(a) What would you predict would happen when equimolar amounts of NaN 3 and NaNO 2 react...
Question:
(a) What would you predict would happen when equimolar amounts of NaN3 and NaNO2 react in acidic solution? How would you attempt to confirm your prediction?
(b) POCl3 reacts with an excess of Me2NH to yield compound A as the only phosphorus-containing product; compound A is miscible with water. A contains 40.21%C, 23.45%N and 10.12%H, and each of the solution 1H and 13C NMR spectra exhibits one signal. Equimolar amounts of A and RNH2 (R = alkyl) react, eliminating dimethylamine to give B (shown below).
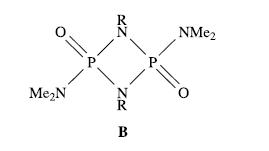
(i) Suggest the identity of A, and draw its structure, giving a resonance form in which all non-H atoms obey the octet rule.
(ii) What is the origin of the miscibility of A with water?
(iii) Write a balanced equation for the formation of A from POCl3 and Me2NH.
Step by Step Answer:






