Among the naturally occurring minerals of Al are diaspore (-AlO(OH)), boehmite (-AlO(OH)) and gibbsite (-Al(OH) 3 ).
Question:
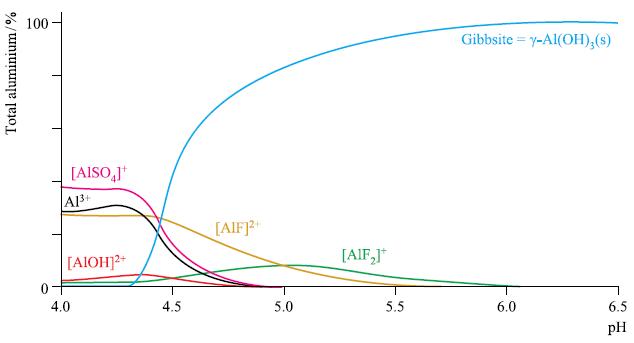
Among the naturally occurring minerals of Al are diaspore (α-AlO(OH)), boehmite (γ-AlO(OH)) and gibbsite (γ-Al(OH)3). At low pH, reactions with H+ give rise to water-soluble [Al(OH2)6]3+, abbreviated to Al3+ (aq). Thus, Al becomes mobile in rivers and other water courses, but the species present are pH dependent and also depend on other inorganic ions in solution. Using known stability constants, the speciation has been modelled over a pH range of 4.0 to 6.5 and with sulfate and fluoride ions present (Fig. 7.14).
(a) What can you deduce about values of Ksp for diaspore, boehmite and gibbsite?
(b) Draw the structures of the species present in solution at pH 4.5.
(c) In the absence of [SO4]2−and F‾, Al does not enter solution until the pH is lowered to ≈ 4.8. Suggest likely forms in which the Al is present.
(d) Write equilibria that describe the relationships between the curves drawn in Fig. 7.14 for Al3+ and [AlOH]2+, and for [AlF]2+ and [AlF2]+.
(e) For the reaction of Al3+ (aq) with F‾, log β1 = 7.0, and log β2 = 12.7. To what equilibria do these values refer? Determine values of K1 and K2 for appropriate stepwise reactions of Al3+ (aq) with F‾.
(f) Give a qualitative explanation for the shapes of the curves in Fig. 7.14.
Figure 7.14
Step by Step Answer:






