By considering the reactions 8E(g) 4E 2 (g) and 8E(g) E 8 (g) for E
Question:
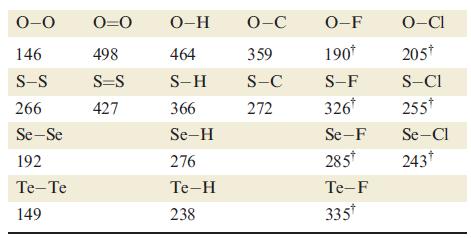
By considering the reactions 8E(g) → 4E2(g) and 8E(g) → E8(g) for E = O and E = S, show that the formation of diatomic molecules is favoured for oxygen, whereas ring formation is favoured for sulfur. [Data: see Table 16.2.]
Table 16.2.
Transcribed Image Text:
0-0 146 S-S 266 Se-Se 192 Te-Te 149 0=0 498 S=S 427 O-H 464 S-H 366 Se-H 276 Te-H 238 O-C 359 S-C 272 O-F 190* S-F 326* Se-F 285* Te-F 335* O-CI 205* S-CI 255* Se-Cl 243†
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (4 reviews)
To determine whether the formation of diatomic molecules or ring formation is favored for oxygen O and sulfur S we need to compare the enthalpy changes H for the two reactions 8Eg 4E2g and 8Eg E8g for E O and E S Lets consider the data from Table 162 for oxygen O and sulfur S For oxygen O Enthalpy change ...View the full answer

Answered By

Surendar Kumaradevan
I have worked with both teachers and students to offer specialized help with everything from grammar and vocabulary to challenging problem-solving in a range of academic disciplines. For each student's specific needs, I can offer explanations, examples, and practice tasks that will help them better understand complex ideas and develop their skills.
I employ a range of techniques and resources in my engaged, interesting tutoring sessions to keep students motivated and on task. I have the tools necessary to offer students the support and direction they require in order to achieve, whether they need assistance with their homework, test preparation, or simply want to hone their skills in a particular subject area.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Use the runs test to determine whether the sample is random. Let alpha be.05. 2 2 2 2 2 1 2 2 2 1 1 2 2 2
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family. The Incisors own a rental beach house in Hawaii. The beach house was rented for the full year during 2012...
-
John works in a factory as a Quality Expert and is responsible for doing quality product testing before the finished products are sent to the customers. John takes a sample of 10 from a batch of 1000...
-
Under a perpetual inventory system, a company should know the quantity and price of its inventory at any moment in time. Given this, why do companies that use a perpetual inventory system still take...
-
The warping effect of the bilinear transformation also affects the phase of the transformed filter. Consider a filter with transfer function G(s) = e 5s . (a) Find the transformed discrete...
-
Repeat the calculations of Example 9.5, but for a total solution normality of 0.5. Data From Example 9.5:- For the Cu 2+ /Na + exchange with a strong-acid resin, show how the fraction CuR2 in the...
-
The following items (in millions) pertain to Calendar Corporation RequiredCalendar's manufacturing costing system uses a three-part classification of direct materials, direct manufacturing labor, and...
-
Lindal Corporation, organized in 2023, immediately filed an election for S corporation status under the rules of Subchapter S. What is the maximum amount of passive investment income that Lindal will...
-
(a) Use the values of E for reactions 16.32 and 16.33 to show that H 2 O 2 is thermodynamically unstable with respect to decomposition into H 2 O and O 2 . (b) 20 Volume H 2 O 2 is so called because...
-
Polyphosphazenes are an important class of inorganic macromolecule and have many commercial applications, e.g. fire retardants, elastomers, fuel cell membranes, biomedical applications. The scheme...
-
For the linkage shown, determine the force Q required for equilibrium when l =18 in., M = 600 lb in., and = 70. B,
-
in a linear search algorithm, what is the worst - case scenario? 3 6 3 6 A . . When key is the last element in array B . . When key is not found in array C . . When key is the first element in array...
-
The output of the code at the end is given in the line below: 01234012 Complete the missing part in the following python code to have an output given in the line above (Do not use extra spaces) for x...
-
Which of these is a traffic exchange point in the routing hierarchy of the Internet that connects national service providers? Peer network Network access point Regional service provider Wireless...
-
Questions: 1. Explain 'Local Variable' and 'Instance variable'. Show theirs scoop on an example java program.
-
A _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ refers to a large computer network that spans a city. MAN WAN LAN PAN
-
Draw a box plot for the data in problem 1-47. Are there any outliers? Is the distribution of the data symmetric or skewed? If it is skewed, to what side? In problem 7.0, 6.9, 8.2, 7.8, 7.7, 7.3, 6.8,...
-
One hundred pounds of water at atmospheric pressure are heated from 60F to 200F. What is the enthalpy change? The internal energy change? Why is the difference between the internal energy change and...
-
Predict the shape of the doubly chlorine-bridged I 2 Cl 6 molecule by using the VSEPR model, and assign the point group.
-
(a) Use the VSEPR model to predict the probable shapes of [IF 6 ] + and IF 7 . (b) Give a plausible chemical equation for the preparation of [IF 6 ][SbF 6 ].
-
Sketch all the isomers of the complexes [CrCl 4 F 2 ] 3 and [CrCl 3 F 3 ] 3 . Indicate how many fluorine environments would be indicated in the 19 F-NMR spectrum of each isomer.
-
Larry's friend, Leon, has decided to buy shares in Larry's new coffee shop, Latte Larry's. The current market price of the stock is $20. Over the next year, Leon expects the company to pay dividends...
-
For a particular reaction at 300 K, AG = -600 kJ/mol, and AS = 0.4 kJ/(K mol). What is the AG for this reaction at 100 K? (Round to the nearest integer. IMPORTANT: when entering a negative value, do...
-
Equilibrium phase transitions occur under constant temperature and constant pressure conditions. Condensation describes the phase transition from a gas to a liquid. What is the sign of delta H for...

Study smarter with the SolutionInn App


