Carbon monoxide is a toxic pollutant which arises from the partial combustion of carbon-based fuels. Complete combustion
Question:
Carbon monoxide is a toxic pollutant which arises from the partial combustion of carbon-based fuels. Complete combustion produces CO2. The toxicity of CO is a result of its competition for the O2-binding sites in blood, i.e. the iron present in haemoglobin (see Chapter 29). When CO binds to the iron, it prevents O2 from being carried in the bloodstream. The following are resonance structures for CO:
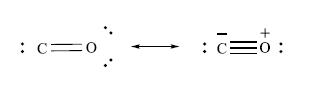
(a) Comment on these structures in terms of the octet rule.
(b) How is the right-hand resonance structure related to a Lewis structure for N2?
(c) A primary interaction between CO and iron in haemoglobin involves a lone pair of electrons on the carbon atom. Using MO theory, explain how this lone pair arises.
(d) Without treatment, severe CO poisoning is fatal. Explain why a hyperbaric chamber containing pure O2 at a pressure of 1.4 bar is used to treat a patient with severe CO poisoning. Normal air contains 21% O2.
Step by Step Answer:






