Consider the half-reaction: If the ratio of concentrations of [MnO 4 ] : Mn 2+ is
Question:
Consider the half-reaction:
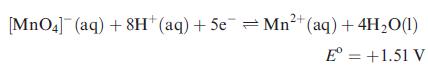
If the ratio of concentrations of [MnO4]− : Mn2+ is 100:1, determine E at pH values of
(a) 0.5;
(b) 2.0;
(c) 3.5 (T = 298 K).
Over this pH range, how does the ability of permanganate(VII) (when being reduced to Mn2+) to oxidize aqueous chloride, bromide or iodide ions change?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





