Consider the molecular orbital diagram for a tetrahedral complex (based on Fig. 20.8) and the relevant d-orbital
Question:
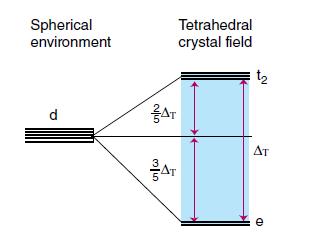
Consider the molecular orbital diagram for a tetrahedral complex (based on Fig. 20.8) and the relevant d-orbital configuration, and show that the purple colour of [MnO4]− ions cannot arise from a ligand-field transition. Given that the wavenumbers of the two transitions in [MnO4]− are 18 500 and 32 200 cm−1, explain how to estimate ΔT from an assignment of the two charge-transfer transitions, even though ΔT cannot be observed from a direct d-d transition.
Figure 20.8.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Inorganic Chemistry
ISBN: 9780198768128
7th Edition
Authors: Mark Weller, Tina Overton, Jonathan Rourke
Question Posted:





