Fluoride ions are added to drinking water, toothpaste and drugs used to treat osteoporosis. However, an excess
Question:
Fluoride ions are added to drinking water, toothpaste and drugs used to treat osteoporosis. However, an excess of F− is toxic to the body. The design of water-soluble, selective fluoride ion receptors is therefore topical. One group of receptors that has been investigated consists of the boron-containing species shown below (Mes = mesityl = 2,4,6-Me3C6H2):
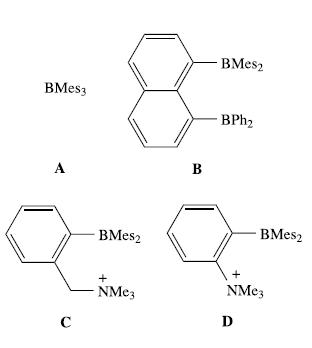
(a) Explain in detail how compound A binds F−.
(b) Suggest why the stability constant of the complex formed between B and F− is greater than that formed between A and F−.
(c) Compounds C and D form zwitter-ionic complexes with F−. Explain what this means.
(d) The 1H NMR spectrum of the complex formed between C and F− shows two signals for the CH2 protons: δ 3.82 (d, JHH = 12.9 Hz) and 6.50 (dd, JHH = 12.9 Hz, JHF = 9.2 Hz). Draw the structure for a complex that is consistent with this observation, and also explains the high stability of the complex.
(e) Which of the receptors would you expect to form water-soluble complexes with F−?
Step by Step Answer:






