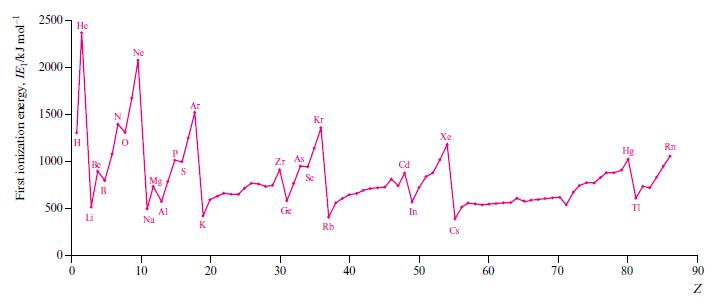
In Fig. 1.16, identify the trends in the first ionization energies of the elements in (a) Descending
Question:
In Fig. 1.16, identify the trends in the first ionization energies of the elements in
(a) Descending group 1,
(b) Descending group 13,
(c) Crossing the first row of the d-block,
(d) Crossing the row of elements from B to Ne,
(e) Going from Xe to Cs,
(f ) Going from P to S.
Rationalize each of the trends you have described.
Figure 1.16
Transcribed Image Text:
First ionization energy, IE,/kJ mol 2500 He 2000- 1500- 1000- 500- H Li B Mg Na 10 Al 20 Zr As 30 Kr Se Rb T 40 Cd In 50 Xe 60 70 Hg 11 80 Rn - N 90
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (6 reviews)
In Figure 116 the trends in the first ionization energies of the elements can be observed The xaxis represents the atomic number Z and the yaxis repre...View the full answer

Answered By

User l_998468
I have extensive tutoring experience, having worked as a private tutor for over three years. I have tutored students from different academic levels, including high school, undergraduate, and graduate levels. My tutoring experience has taught me to be patient, attentive to student needs, and effective in communicating difficult concepts in simple terms.
I have a strong background in statistics, probability theory, data analysis, and data visualization. I am proficient in using statistical software such as R, Python, and SPSS, which are commonly used in academic research and data analysis. Additionally, I have excellent communication and interpersonal skills, which enable me to establish rapport with students, understand their learning styles, and adapt my teaching approach to meet their needs.
I am passionate about teaching and helping students achieve their academic goals.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Potassium and hydrogen react to form the ionic compound potassium hydride. (a) Write a balanced equation for this reaction. (b) Use data in Figures 7.10 and 7.12 to determine the energy change in...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-7. Ivan sold the following securities during the year and received a Form 1099-B that...
-
For the following exercises, use shells to find the volume generated by rotating the regions between the given curve and y = 0 around the x-axis. 130. y = 1-x,x = 0, and x = 1 131. y = x, x = 0, and...
-
IPW, Inc., began operations on January 1, 2012. The seven transactions recorded during January by the company accountant are shown in the following T-accounts: Complete the following table. For each...
-
Let Z Gamma(a, ), where a is an integer. Conditional on Z = z, let X Pois(z). Show that X has a negative binomial distribution with the following interpretation. For k = 0, 1, . . . , P(X = k) is...
-
In 1970, Rose Mary Knick purchased 90 acres of land in Scott Township, Lackawanna County, Pennsylvania. In 2008, another resident of Scott Township discovered documents that suggested that one of...
-
The capital structure of Blacksmith, Inc., at December 31, 2011, included 18,000 shares of $1 preferred stock and 38,000 shares of common stock . Common stock outstanding during 2012 totaled 38,000...
-
ces Direct labor-hours Machine-hours Fixed manufacturing overhead cost Variable manufacturing overhead per machine-hour Variable manufacturing overhead per direct labor-hour 35,000 Department Molding...
-
Calculate the energy of the 3s atomic orbital of an H atom. Is the energy of the hydrogen 3p atomic orbital the same as or different from that of the 3s orbital?
-
Which of the following species are hydrogen-like: (a) H + ; (b) He + ; (c) He ; (d) Li + ; (e) Li 2+
-
Let A be an m n matrix with singular value decomposition UVT and suppose that A has rank r, where r < n. Let b Rm. Show that a vector x Rn minimizes ||b - Ax||2 if and only if x = A+b + cr+1vr+1 +...
-
The Bureau of Labor Statistics (BLS) tracks the numbers of workers who are employed part-time for economic reasons. The number typically increases sharply at the beginnings of recessions and...
-
The natural rate of unemployment is higher in France than in the United States. Suppose you are a recent college graduate and you are eager to find a job. Which countrys labor market seems more...
-
What is the time-inconsistency problem, and what role does it play in the debate between advocates of discretion and advocates of rules in policy making?
-
Suppose the U.S. Congress is forced to increase taxes to pay for the cost of health care reform in the United States. Describe the effects of such a policy, according to the three business cycle...
-
What is the real interest rate? Why can the Fed control the real interest rate in the short run but not in the long run?
-
Suppose you are the general manager of a company with approximately 100 employees and the government has just announced its new rates for contributions to the Canada Pension Plan and Employment...
-
ABC company leased new advanced computer equipment to STU Ltd on 1 January 2019.STULtd has to pay annual rental of $290,000 starting at 1 January 2019. It is a four years lease with ultimate rental...
-
Identify the incorrect phrase in each of the following statements; explain your answer in each case. (a) Sodium dissolves in ammonia and amines to produce the sodium cation and solvated electrons or...
-
Which of the following pairs is most likely to form the desired compound? Describe the periodic trend and the physical basis for your answer in each case. (a) Ethanoate ion or edta 4 ion to react...
-
Summarize the chemistry of sodium that is being researched for the development of rechargeable sodium ion batteries. See M.D. Slater, D Kim, E. Lee, and C.S. Johnson, Adv. Func. Mater., 2013, 23, 947.
-
Find x-component of a = (1.3 m/s, 30 above the negative x-axis). Express your answer with the appropriate units. View Available Hint(s) = Value Submit Part D 0 ? Units Find y-component of a = (1.3...
-
How high is the image of the insect if the focal length of the lens is f = -125 mm? Follow the sign conventions. Express your answer to three significant figures and include the appropriate units....
-
ns The building slab is subjected to four parallel column loadings. Take F = 8 kN, and F = 9 kN. Part A 6kN Z 12 kN 6 m 16 m 12 m 4 m Determine the magnitude of the equivalent resultant force....

Study smarter with the SolutionInn App


