In the traditional scheme for the separation of metal ions from solution that is the basis of
Question:
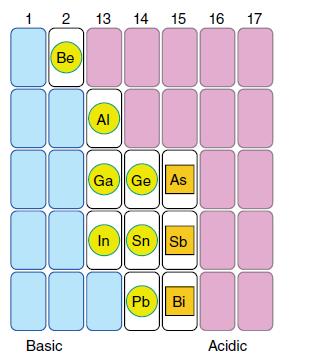
In the traditional scheme for the separation of metal ions from solution that is the basis of qualitative analysis, ions of Au, As, Sb, and Sn precipitate as sulfides but redissolve on addition of excess ammonium polysulfide. By contrast, ions of Cu, Pb, Hg, Bi, and Cd precipitate as sulfides but do not redissolve. In the language of this chapter, the first group is amphoteric for reactions involving SH− in place of OH−. The second group is less acidic. Locate the amphoteric boundary in the periodic table for sulfides implied by this information. Compare this boundary with the amphoteric boundary for hydrous oxides in Fig. 5.5. Does this analysis agree with describing S2− as a softer base than O2−?
Figure 5.5.
Step by Step Answer:

Inorganic Chemistry
ISBN: 9780198768128
7th Edition
Authors: Mark Weller, Tina Overton, Jonathan Rourke





