NaAlH 4 is among lightweight metal hydrides being investigated as a means of storing hydrogen, e.g. for
Question:
NaAlH4 is among lightweight metal hydrides being investigated as a means of storing hydrogen, e.g. for fuel cell applications. Decomposition occurs in three steps upon heating:
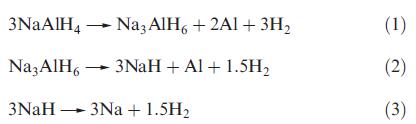
(a) Calculate the H content of NaAlH4 as a wt %.
(b) Step (3) occurs above 670K and this limits the practical dehydrogenation steps to (1) and (2). What is the hydrogen storage capacity (in wt %) of NaAlH4 if only steps (1) and (2) are considered?
(c) Unfortunately, the kinetics of the dehydrogenation of NaAlH4 militate against practical applications as a hydrogen storage material, but doping the material with Ti improves the kinetics both of dehydrogenation and rehydrogenation. Comment on this statement in terms of the role of the dopant, and the need for both dehydrogenation and rehydrogenation to be viable processes.
Step by Step Answer:






