Reduction potentials for half-cell reactions of simple carbon species measured in aqueous solution at pH 7.0, 25C,
Question:
Reduction potentials for half-cell reactions of simple carbon species measured in aqueous solution at pH 7.0, 25°C, are as follows.
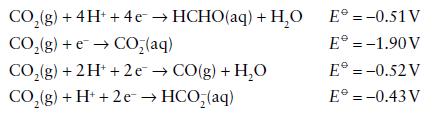
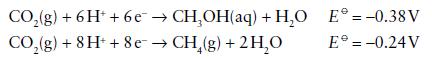
(a) Assign carbon oxidation numbers to each of the species involved.
(b) Construct a Frost diagram, making HCHO the reference point at the origin.
(c) Identify the redox couple having the most positive reduction potential.
(d) Is hydration of CO to give formate (HCO2−) favourable at pH 7.0?
(e) At pH 7.0, 25°C, the reduction potential for the hydrogen half-cell is given by:
![]()
Calculate the equilibrium constant for the water-gas shift reaction
![]()
in aqueous solution, under these conditions.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Inorganic Chemistry
ISBN: 9780198768128
7th Edition
Authors: Mark Weller, Tina Overton, Jonathan Rourke
Question Posted:





