Refer to Table 6.2. (a) Write an equation for the process for which the standard enthalpy of
Question:
Refer to Table 6.2.
(a) Write an equation for the process for which the standard enthalpy of atomization of cobalt is defined.
(b) Suggest reasons for the trend in standard enthalpies of atomization on descending group 1.
(c) Outline possible reasons for the trend in values of ∆aH° on going from Cs to Bi.
Table 6.2.
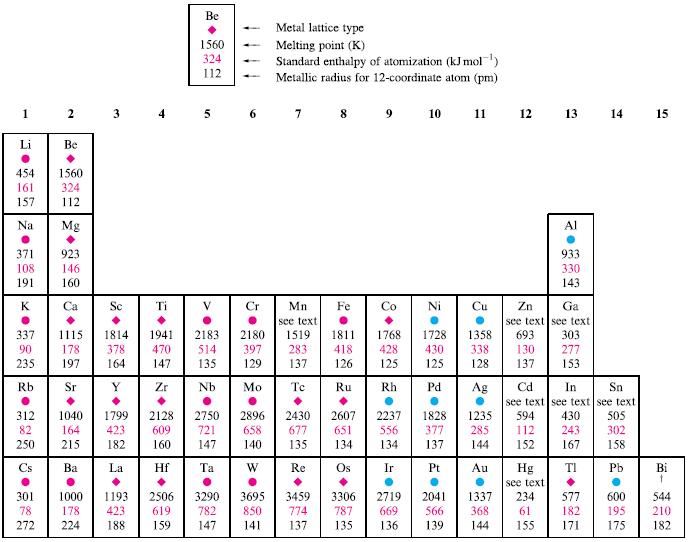
Transcribed Image Text:
Li 1 2 3 4 5 454 161 157 Na 371 108 K 337 90 235 Cs Be 301 78 1560 324 112 Mg 923 146 Rb Sr 312 1040 1799 DE 82 250 Ba Sc 1115 1814 178 378 470 197 164 147 Zr 164 423 215 182 La 178 423 188 272 224 Ti Be 1560 324 112 1941 2183 2180 514 135 Nb Hf 2128 2750 609 721 147 Ta 3290 782 619 159 147 6 7 8 9 10 11 12 13 14 397 129 Mo Metal lattice type Melting point (K) Standard enthalpy of atomization (kJ mol-¹) Metallic radius for 12-coordinate atom (pm) 2896 658 140 W Mn see text 1519 283 137 Tc 2430 677 135 Re Fe 850 774 141 1811 1768 418 428 126 125 Ru Rh 2607 651 134 Os 3306 787 137 135 2237 556 134 Ni 1728 1358 430 338 125 128 Pd. Ag 1828 1235 377 137 Pt 144 Au 2041 566 368 669 136 139 Zn see text 693 130 137 Hg see text 234 61 Al Cd In Sn see text see text see text 594 430 505 112 243 302 152 167 158 TI Pb 144 155 933 330 143 Ga see text 303 277 153. 577 182 15 Bi t 544 195 210 171 175 182
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (3 reviews)
a The process for which the standard enthalpy of atomization of cobalt is defined is Cog Co atomsg In this process one mole of gaseous cobalt atoms is ...View the full answer

Answered By

Diana Muriuki
As an online math tutor, I have several years of hands-on experience working with students of all ages and skill levels. I hold a Bachelor's degree in Mathematics and a Master's degree in Education. Additionally, I have completed multiple training courses in online teaching and tutoring methods.
Throughout my career, I have worked with students in both individual and group settings, including classroom teaching, after-school tutoring, and online instruction. I am proficient in teaching a wide range of math topics, from basic arithmetic to advanced calculus and statistics.
One of my greatest strengths as a tutor is my ability to adapt my teaching style to meet the unique needs and learning styles of each individual student. I understand that every student is different, and I strive to create a comfortable and supportive learning environment that encourages growth and development.
In addition to my formal education and tutoring experience, I am also a lifelong learner with a passion for mathematics. I am constantly seeking out new resources and methods to improve my own knowledge and skills, and I believe this passion and enthusiasm helps to inspire my students as well.
Overall, my hands-on experience and proficiency as a math tutor are grounded in a combination of formal education, practical experience, and a genuine love of mathematics. I am confident in my ability to help students achieve their goals and succeed in math, and I look forward to the opportunity to work with new students and continue to grow as an educator.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Write an equation for the process that corresponds to the electron affinity of the ion. Also write the electron configurations of the species involved. What is the magnitude of the energy change in...
-
The standard enthalpy of atomization of an element is the energy required to convert one mole of an element in its most stable form at 25C to one mole of monatomic gas. Given that the standard...
-
Write an equation for the reaction of hydrazine with fluorine gas to produce nitrogen gas and hydrogen fluoride gas. Estimate ÎH for this reaction, using bond energies from Table 13.6. Table...
-
1-Calculate amount of secondary compression in terms of inches 10 years following end of the primary consolidation. S.sc ang (12)= Coefficient of secondary compression=0.035 Thickness of clay layer-...
-
Regarding the two countries with the largest labor force shown on PengAtlas Map 4.3, what advantage does the large size provide? What is the disadvantage?
-
Based on all three estimates and on the valuation figures for the three competitors how much per share do you think that Orange Brite is really worth? Explain your rationale.
-
North Shore Architectural Stone, Inc., a company that installs limestone in residential and commercial buildings, agreed to supply and install limestone for a property owned by Joseph Vitacco. North...
-
Dallas Industries has adopted the following production budget for the first 4 months of 2014. Each unit requires 2 pounds of raw materials costing $2 per pound. On December 31, 2013, the ending raw...
-
Suppose that both players discount future payoffs with the same discount factor ? < 1. Suppose that both players play the "Cooperative Strategy;" namely, they play C in every period, no matter what...
-
(a) Draw a set of resonance structures for the hypothetical molecule PH 5 , ensuring that P obeys the octet rule in each structure. Assume a structure analogous to that of PF 5 . (b) To what point...
-
(a) What hybridization scheme would be appropriate for the Si atom in SiH 4 ? (b) To which point group does SiH 4 belong? (c) Sketch a qualitative MO diagram for the formation of SiH 4 from Si and an...
-
Madacy Entertainment Inc. showed the following equity account balances on the December 31, 2013, balance sheet: Common shares, unlimited authorized shares, 680,000 shares issued and...
-
Why does the future value of a given amount increase when interest is compounded nonannually as opposed to annually?
-
Why might a very high current ratio actually indicate theres a problem with a firms inventory or accounts receivable management? What might be another reason for a high current ratio?
-
Suppose you are a financial advisor and a corporate treasurer at a corporation, which is going to issue a new security. The corporation requires your expertise on flotation costs. In this context,...
-
What is the relationship between the interest rate and number of years on an amortized loan and the total amount of interest paid?
-
Explain the formula FVn = PV(1 + r)n.
-
Slick Snowboards Company reported sales of $700,000 in the first quarter of 2016. The company has never implemented an inventory system so the controller does not know how much inventory is actually...
-
Chapter 9 Stock Valuation at Ragan Engines Input area: Shares owned by each sibling Ragan EPS Dividend to each sibling Ragan ROE Ragan required return Blue Ribband Motors Corp. Bon Voyage Marine,...
-
Interpret the variation, including the overall trend across the 3d series, of the following values of oxide lattice enthalpies (in kJ mol 1 ). All the compounds have the rock-salt structure: CaO...
-
For each of the following pairs of complexes, identify the one that has the larger LFSE: (a) [Cr(OH 2 ) 6 ] 2+ or [Mn(OH 2 ) 6 ] 2+ (b) [Fe(OH 2 ) 6 ] 2+ or [Fe(OH 2 ) 6 ] 3+ (c) [Fe(OH2) 6 ] 3+ or...
-
Solutions of the complexes [Co(NH 3 ) 6 ] 2+ , [Co(OH 2 ) 6 ] 2+ (both O h ), and [CoCl 4 ] 2 are coloured. One is pink (absorbs blue light), another is yellow (absorbs violet light), and the third...
-
x+5 x-2 Subtract and simplify: x-3 x+7
-
Exercise 2 (Total: 20 points) Suppose a hypothetical study analyzes the factors that explain the extent of extramarital affairs in a marriage, and estimates the following multiple linear regression...
-
2. Darrell spoke with a financial advisor and learns he can afford to make monthly payments of $1000 for the next 5 years in order to start a business. At this point in time, he is able to borrow an...

Study smarter with the SolutionInn App


