The hydrido complex [FeH 6 ] 4 has Oh symmetry. The bonding in [FeH 6 ]
Question:
The hydrido complex [FeH6]4‾ has Oh symmetry. The bonding in [FeH6]4– can be described in terms of the interactions between the atomic orbitals of Fe and the LGOs of the H6-fragment.
(a) Derive the six LGOs of the H6 fragment, showing clearly how you determine their symmetries.
(b) The basis set for the Fe atom consists of valence 3d (see Fig. 1.12), 4s and 4p orbitals. Determine the symmetries of these orbitals under Oh symmetry.
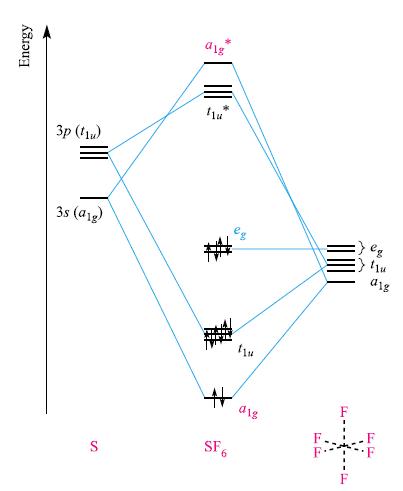
(c) Construct an MO diagram for the formation of [FeH6]4‾ from Fe and the H6-fragment, showing which MOs are occupied. Comment on the characters of the MOs. How does this bonding picture differ from that described for SF6 in Fig. 5.28?
Figure 1.12.
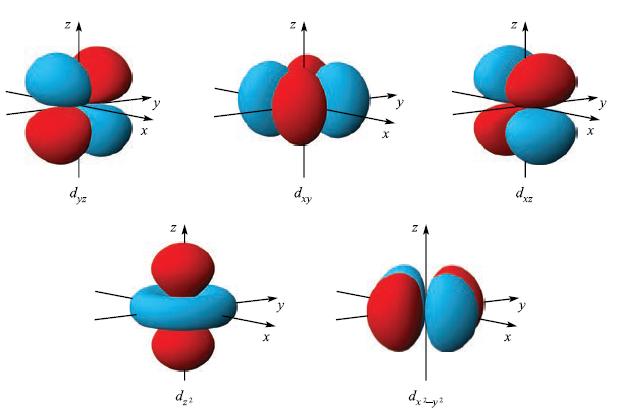
Figure 5.28.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





