The tanning process in the manufacture of leather relies upon the interaction of Cr 3+ with the
Question:
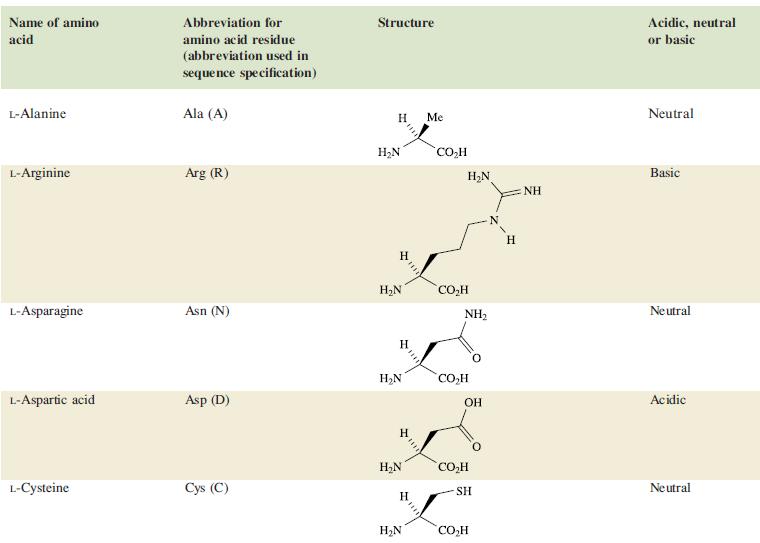
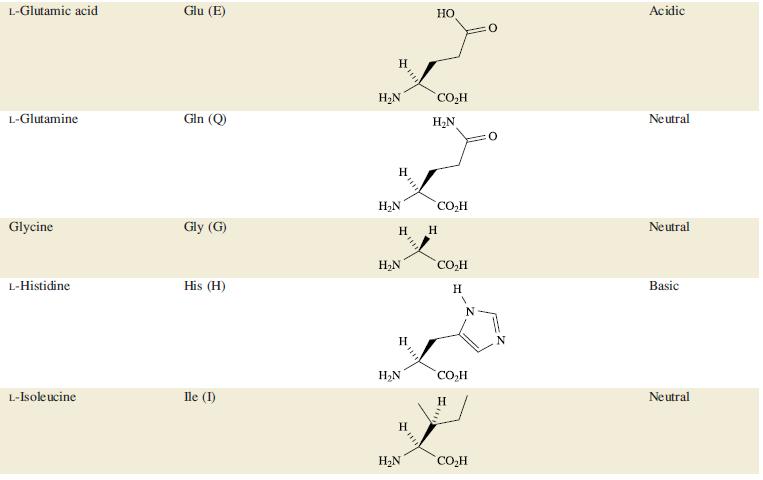
The tanning process in the manufacture of leather relies upon the interaction of Cr3+ with the fibrous protein collagen. Although glycine and L-proline are the most important amino acids (see Table 29.2) in collagen, glutamic acid (pKa = 3.8) and aspartic acid (pKa = 4.2) are also present. During tanning, the pH of an aqueous solution of Cr(OH)SO4 is lowered from ≈2.8 to 3.8.
(a) What chromium(III) ion is present in aqueous solution at very low pH and why is H+ needed to stabilize this species?
(b) In the absence of collagen, aqueous solutions of Cr3+ at pH 3.8 contain linear, tri- and tetranuclear species. Suggest structures for these species.
(c) A study [A.D. Covington et al. (2001) Polyhedron, vol. 20, p. 461] of the interaction of chromium(III) with collagen states that, at pH 3.8, carboxylate groups should compete with hydroxido ligands for chromium. The study concludes that the predominant chromium-containing species bound to leather is a linear oligomer with nuclearity 2 or 3. Using this information, suggest how Cr3+ interacts with collagen and indicate how Cr3+ may be involved in cross-linking of collagen fibres.
(d) A major concern in the tanning industry is to prevent toxic waste arising from the oxidation of Cr(III) to Cr(VI). Chromium(VI) may be present as [Cr2O7]2− and two other anions, depending upon the pH. Write equilibria to show how the three Cr(VI) species are interrelated, and suggest a method to remove them from waste water.
Table 29.2.
Step by Step Answer:






