Typical compositions of nickelmetal hydride (NiMH) batteries are shown below: (a) Write equations to show the processes
Question:
Typical compositions of nickel–metal hydride (NiMH) batteries are shown below:
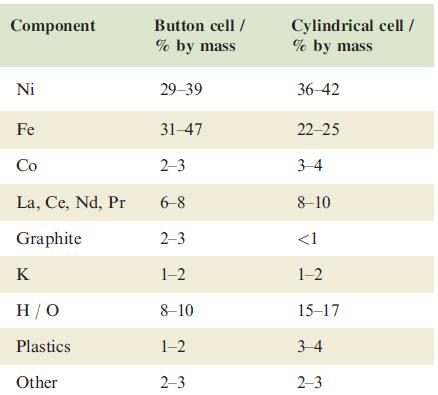
(a) Write equations to show the processes at the anode and cathode during charging and discharging a NiMH cell.
(b) What is the overall cell reaction? Confirm that the changes in oxidation states for reduction and oxidation reactions balance.
(c) What role does the mixture of f-block elements in the battery play?
(d) Why is Fe needed in the battery?
(e) During battery recycling, suggest a method of recovering Co and Ni.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





