Use the data in Appendix 11 to rationalize the following observations in a quantitative manner. What assumption(s)
Question:
Use the data in Appendix 11 to rationalize the following observations in a quantitative manner. What assumption(s) have you made in answering this question?
(a) The dithionate ion, [S2O6]2−, can be prepared by controlled oxidation of [SO3]2− using MnO2.
(b) In the presence of acid, KI and KIO3 react to form I2.
(c) Mn2+ is instantly oxidized to [MnO4]− by aqueous solutions of H4XeO6.
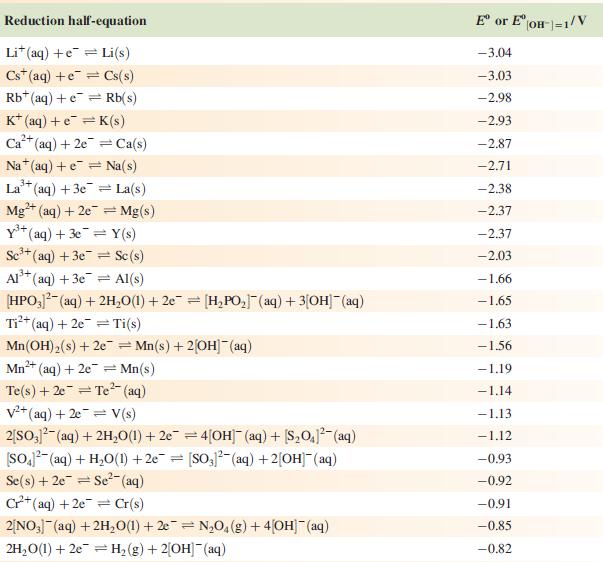
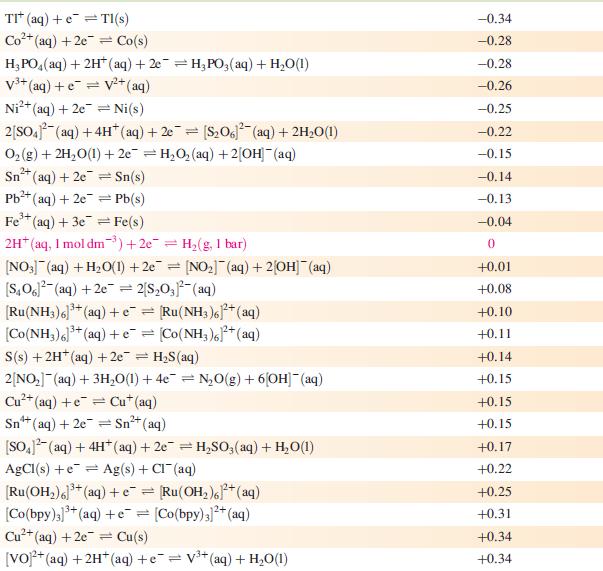
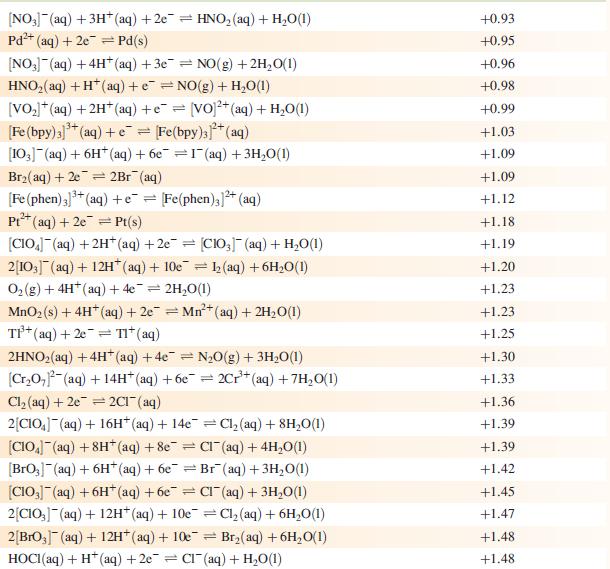
Data from Appendix 11
The concentration of each aqueous solution is 1 mol dm−3 and the pressure of a gaseous component is 1 bar (105 Pa). (Changing the standard pressure to 1 atm (101 300 Pa) makes no difference to the values of Eo at this level of accuracy.) Each half-cell listed contains the specified solution species at a concentration of 1 mol dm−3; where the half-cell contains [OH]−, the value of Eo refers to [OH−] = 1 mol dm−3, hence the notation Eo[OH−] = 1

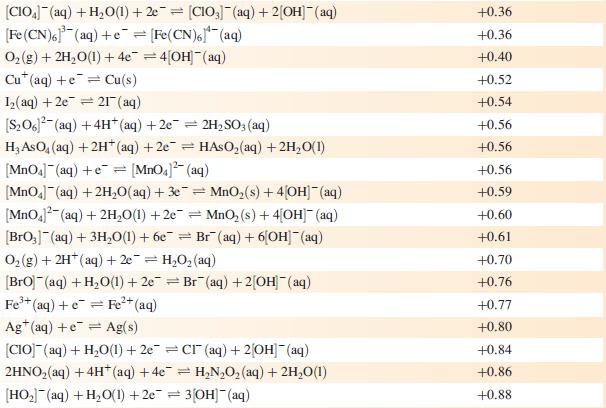

Step by Step Answer:






