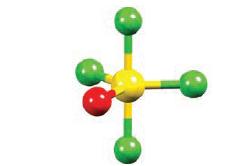
Use the VSEPR model to rationalize the structure of SOF 4 shown in Fig. 2.19. What are
Question:
Use the VSEPR model to rationalize the structure of SOF4 shown in Fig. 2.19. What are the bond orders of
(a) Each S—F bond
(b) The S—O bond?
Figure 2.19
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (6 reviews)
In the VSEPR Valence Shell Electron Pair Repulsion model the electron pairs around a central atom ar...View the full answer

Answered By

Chandrasekhar Karri
I have tutored students in accounting at the high school and college levels. I have developed strong teaching methods, which allow me to effectively explain complex accounting concepts to students. Additionally, I am committed to helping students reach their academic goals and providing them with the necessary tools to succeed.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The reaction of three molecules of fluorine gas with a Xe atom produces the substance xenon hexafluoride, XeF6: Xe(g) + 3 F2(g) XeF6(s) (a) Draw a Lewis structure for XeF6. (b) If you try to use the...
-
(a) Write down the ions that are present in the compound [PCl 4 ][PCl 3 F 3 ]. What shape do you expect each ion to adopt? In theory, does either ion possess stereoisomers? (b) Use the VSEPR model to...
-
Use the VSEPR model to predict the geometry of the following ions: a. N3 b. BH4 c. SO32 d. NO2
-
6- Where is "bunds Payable" account recorded in the balance sheet? A)Long Term Liabilities B) Non Current Assets C) Current Assets D) Owner's Equity
-
This problem continues with the business of Pure Water, Inc., begun in the continuing problem in Chapter 1. Here you will account for Pure Water, Inc.s transactions using formal accounting practices....
-
In this chapter, we modeled growth in an economy by a growing population. We could also achieve a growing economy by having an endowment that increases over time. To see this, consider the following...
-
Im gathering some information about the sales/collection process and how it is supposed to work. Okay?
-
Tiffany Martin is an audit manager in a medium-sized public accounting firm. Tiffany graduated from college seven years ago with a degree in accounting. She obtained her CPA certification soon after...
-
A square insulating plate, 2 meters by 2 meters, lies flat on the floor. A total charge Q = 8 10-8 C is distributed uniformly on the sheet. A pith ball (a small non-conducting ball made of a light...
-
(a) Use MO theory to determine the bond order in each of [He 2 ] + and [He 2 ] 2 + . (b) Does the MO picture of the bonding in these ions suggest that they are viable species?
-
Does VB theory indicate that the diatomic molecule He 2 is a viable species? Rationalize your answer.
-
Field Construction agreed to lease payments of $642.79 on construction equipment to be made at the end of each month for three years. Financing is at 9% com- pounded monthly. (a) What is the value of...
-
Rare earth metals are used in the production of many of the personal electronic devices you use every day. Use the interdependence principle to discuss the impact of an unexpected increase in the...
-
In an effort to boost output, the government passes a large fiscal stimulus that raises government purchases by $1 trillion. Use the AD-AS framework to predict how prices and output change in the...
-
Youre a pricing analyst for a manufacturing firm. You are tasked with predicting how average prices will change over the next quarter to help your manager decide how to change her prices. How would...
-
What are the pros and cons of targeting a 0% inflation rate? Do you believe the Fed should target 0% inflation, its current 2% inflation target, or some other value? Explain your reasoning.
-
Explain why the Fed targets inflation rather than employment even though both are part of its dual mandate. Use the interdependence principle to help answer the question.
-
Explain why a financial statement user would want to review a companys contractual commitment note.
-
Briefly discuss the implications of the financial statement presentation project for the reporting of stockholders equity.
-
Show how the coordination of two MeHNCH 2 CH 2 NH 2 ligands to a metal atom in a square-planar complex results in not only cis and trans but also optical isomers. Identify the mirror planes in the...
-
What type of isomerism can arise with ambidentate ligands? Give two examples.
-
The resolving agent d-cis[Co(NO 2 ) 2 (en) 2 ]Br can be converted to the soluble nitrate by grinding in water with AgNO 3 . Outline the use of this species for resolving a racemic mixture of the d...
-
Precision Watches purchases a luxury watch from Germany, including insurance and shipping costs, for $620 per unit. The markup on the watch is 40% for a sales price of $868. Other traceable direct...
-
Total Costs $150000 Total Fixed Costs Unknown Total Variable Costs $89000 Total Revenue $179000 Determine the total fixed costs (in dollars), Determine the profit earned (in dollars), Determine the...
-
How to put in the excel formula for the cost of sales from 2016 with 58% to 2017 cost of sales?

Study smarter with the SolutionInn App


