Butadiene sulfone (MW=118) can be produced by the following irreversible liquid-phase reaction at 190F and 160 psia:
Question:
Butadiene sulfone (MW=118) can be produced by the following irreversible liquid-phase reaction at 190°F and 160 psia:
Butadiene + SO2 → Butadiene Sufone
Pure SO2 is fed to the reactor in one stream. Pure butadiene (MW=54) is fed separately into the reactor at a molar flow rate that is 25% more than that required to react with all of the SO2, and 70% of the entering butadiene is converted into product. The density of the stream leaving the reactor is 42.2 lbm/ ƒt3.
a. For an SO2 flow rate of 100 lbmol/hr, determine the volume of the CSTR required to achieve the specified 70% conversion. The rate of reaction is described by
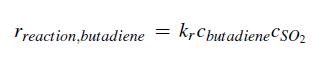
where kr = 4.44 ƒt3/lbmol hr.
b. Would the addition of an inert (non-reacting) liquid to the butadiene feed increase the required reactor size, or decrease it? Why?
Step by Step Answer:

Introduction To Chemical Engineering Tools For Today And Tomorrow
ISBN: 9780470885727
5th Edition
Authors: Kenneth A. Solen, John N. Harb





