Hydrogen gas is a valuable product, because it is used as a feedstock (starting material) for many
Question:
Hydrogen gas is a valuable product, because it is used as a feedstock (starting material) for many chemical processes. A common way to produce high-purity hydrogen gas is by reaction of propane gas with steam using the following scheme:
A. The propane gas is first sent to a Desulfurizer to remove any sulfur present in the propane gas, because the sulfur would poison catalysts in later process steps.
B. Steam is added to the desulfurized propane, and the combined gas is sent to a Reforming Furnace (a fired heater) (1500◦F) to produce the reforming reaction:
![]()
C. More steam is added to the gas mixture leaving the Reforming Furnace, and the combined gas goes to a CO Converter, where the carbon monoxide in the mixture is converted:
![]()
D. The gas mixture from the CO Converter enters the CO2 Absorber, where most of the CO2 in the mixture is absorbed into an amine solution.
E. The gas mixture from the CO2 Absorber now contains H2 with traces of CO and CO2. The last traces of CO and CO2 are converted to methane in a Methanator:
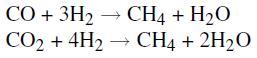
Required
a. Construct a block diagram for the process described above.
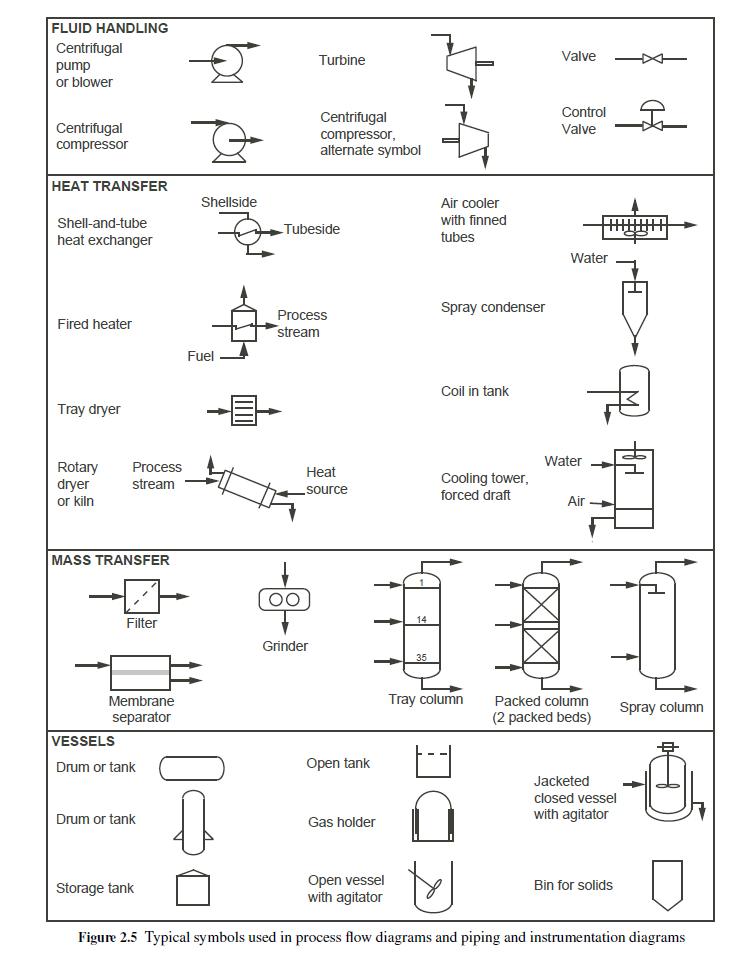
b. Construct a pictorial process flow diagram (without the stream table) using the symbols given in Figure 2.5. The following additional information will be helpful:
 • Liquid propane will be fed froma Propane Tank.
• Liquid propane will be fed froma Propane Tank.
• The propane leaving the Propane Tank is vaporized via a shell-and-tube heat exchanger in which steam is used on the tube side (see the next bullet).
• A shell-and-tube heat exchanger (as you will learn later) is a cylinder (shell) through which a number of tubes pass. One fluid flows inside the tubes (tube side), and the other fluid flows outside the tubes but inside the outer cylinder (shell side), and the streams don't mix. In the symbol for a shell-and-tube heat exchanger, a line representing the tube side passes through the circle (which represents the shell or outer cylinder).
Other lines stop at the boundary of the circle to represent the shell-side fluid entering and leaving the shell. The orientations and directions of the lines and arrows are not critical.
• The Reforming Furnace is a fired heater.
• The Desulfurizer, CO Converter, CO2 Absorber, and Methanator are packed columns with one bed of packing each, and the process gas enters the bottom and exits the top. In the case of the CO2 Absorber, amine solution enters the top (Amine Solution In) and exits the bottom (Amine Solution Out), where the source and destination of the amine solution streams will not be indicated.
• For the CO2 Absorber, the Absorber inlet stream is cooled by two shell-and-tube heat exchangers in series.
In the first exchanger, the Absorber inlet stream flows through the shell side, while cool outlet gas is looped back from the Absorber to flow through the tube side before continuing on its way to the Methanator. In the second exchanger, the Absorber inlet stream again flows through the shell side, while water is used as the tubeside coolant.
• The gas leaving the Methanator is cooled again by water in a shell-and-tube exchanger (the gas on the shell side), and the diagram should label the cooled stream as "Purified hydrogen to storage."
Step by Step Answer:

Introduction To Chemical Engineering Tools For Today And Tomorrow
ISBN: 9780470885727
5th Edition
Authors: Kenneth A. Solen, John N. Harb





