You will remember from your chemistry classes that under the appropriate conditions, gases obey the Perfect Gas
Question:
You will remember from your chemistry classes that under the appropriate conditions, gases obey the “Perfect Gas Law” or “Ideal Gas Law:”
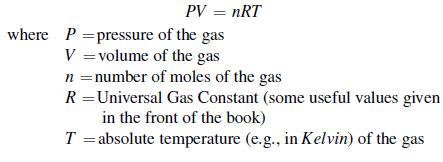
Construct a spreadsheet to graph the volume of 1.0 gmol of a ideal gas at 0°C (273 K) as a function of the pressure. Your graph should show the volume in Liters on the vertical axis and pressure (for a range of 1 to 2 atm with at least 10 intermediate values) on the horizontal axis. Turn in the spreadsheet and the graph.
Step by Step Answer:
Related Book For 

Introduction To Chemical Engineering Tools For Today And Tomorrow
ISBN: 9780470885727
5th Edition
Authors: Kenneth A. Solen, John N. Harb
Question Posted:




