Bubbles tend to form wherever the pressure in a liquid falls below its vapor pressure (P v
Question:
Bubbles tend to form wherever the pressure in a liquid falls below its vapor pressure (Pv), a phenomenon called cavitation. Shock waves created by the rapid collapse of such bubbles can be very damaging to equipment. A system where this might be a concern is shown in Fig. P2.1. To empty a swimming pool for repairs, a pump with a flow rate Q = 3.93 × 10–3 m3/s is to be connected to 50 m of rubber hose with a diameter of 5 cm. The hose is hydrodynamically smooth. The pump intake will be 3 m above the hose inlet, which will rest on the bottom, and the initial water depth will be 2 m. The water properties are as in Table 1.2.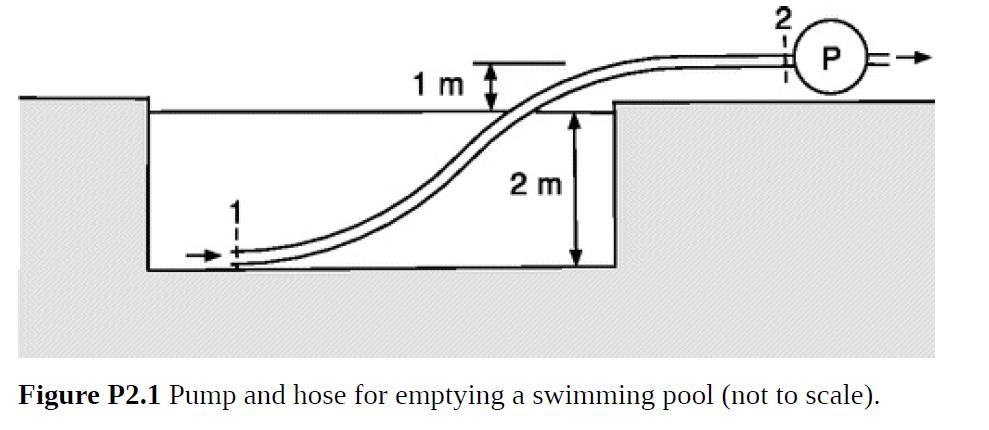
(a) Calculate |Δ℘| from just inside the hose inlet (position 1) to the pump intake (position 2).
(b) Determine P2 when the pool is nearly empty, at which time P1 = 1.01 × 105 Pa. For water at 20 °C, PV = 2339 Pa. You should find that PV < P2 < P0, where P0 is atmospheric pressure.
(c) How much might Q be increased without risking cavitation? Why, at a given Q, does the risk increase as the water level in the pool goes down?
Step by Step Answer:

Introduction To Chemical Engineering Fluid Mechanics
ISBN: 9781107123779
1st Edition
Authors: William M. Deen





