(a) Prove that the entropy of an isolated system either increases or remains constant, but can never...
Question:
(a) Prove that the entropy of an isolated system either increases or remains constant, but can never decrease.
(b) The following figure shows the relationship between temperature and entropy for a closed PVT system during a reversible process. Calculate the heat added to the system for each of the three steps \(1-2,2-3\) and \(3-1\) and for the entire process.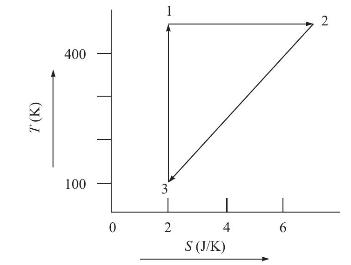
(c) A vessel is divided into two parts and contains \(2 \mathrm{~mol}_{2}\) gas at \(80^{\circ} \mathrm{C}\) and 40 bar on one side and \(3 \mathrm{~mol}\) of argon gas at \(150^{\circ} \mathrm{C}\) and \(15 \mathrm{bar}\) on the other side. Calculate the change in entropy when the partition is removed and the gases are mixed adiabatically and completely. Assume both gases are ideal.
Step by Step Answer:






