A system consisting of a gas confined in a cylinder undergoes a series of processes shown in
Question:
A system consisting of a gas confined in a cylinder undergoes a series of processes shown in Fig. 2.11. During the process A-1-B, \(70 \mathrm{~kJ}\) of heat is added while it does 45 \(\mathrm{kJ}\) of work. Then the system follows the process A-2-B, during which \(55 \mathrm{~kJ}\) of work is performed on the system. How much heat flows into the system during the process \(\mathrm{A}-2-\mathrm{B}\) ? Then the system returns to the initial state along the path B-3-A, and \(80 \mathrm{~kJ}\) of work is done on the system. Calculate the amount of heat transferred between the system and the surroundings during the process B-3-A.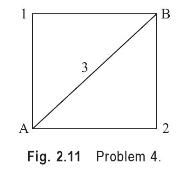
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





