Acetic acid is esterified in the liquid phase with ethanol at (373.15 mathrm{~K}) and (1 mathrm{~atm}) pressure
Question:
Acetic acid is esterified in the liquid phase with ethanol at \(373.15 \mathrm{~K}\) and \(1 \mathrm{~atm}\) pressure to produce ethyl acetate and water according to the reaction
\[ \mathrm{CH}_{3} \mathrm{COOH}(\mathrm{l})+\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{OH}(\mathrm{l}) \rightarrow \mathrm{CH}_{3} \mathrm{COOC}_{2} \mathrm{H}_{5}(\mathrm{l})+\mathrm{H}_{2} \mathrm{O}(\mathrm{l}) \]
If initially there is one mol each of acetic acid and ethanol, estimate the mole fraction
\begin{tabular}{|c|c|c|}
\hline Components & \(\Delta H_{f, 298}^{0}(\mathrm{~kJ})\) & \(\Delta G_{f, 298}^{0}(\mathrm{~kJ})\) \\
\hline \(\mathrm{CH}_{3} \mathrm{COOH}(\mathrm{l})\) & -116.2 & -93.56 \\
\hline \(\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{OH}(\mathrm{l})\) & -66.35 & -41.76 \\
\hline \(\mathrm{CH}_{3} \mathrm{COOC}_{2} \mathrm{H}_{5}(\mathrm{l})\) & -110.72 & -76.11 \\
\hline \(\mathrm{H}_{2} \mathrm{O}(\mathrm{l})\) & -68.31 & -56.68 \\
\hline
\end{tabular}
of ethyl acetate in the reacting mixture at equilibrium by using the following data: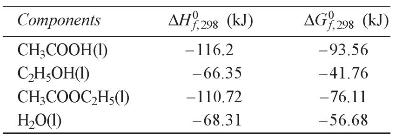
Step by Step Answer:






