Butadiene can be prepared by the gas-phase catalytic dehydrogenation of 1-butene: C 4 H 8 C
Question:
Butadiene can be prepared by the gas-phase catalytic dehydrogenation of 1-butene: C4H8 ⇆ C4H6 + H2. In order to suppress side reactions, the butene is diluted with steam before it passes into the reactor.
(a) Estimate the temperature at which the reactor must be operated in order to convert 30% of the 1-butene to 1,3-butadiene at a reactor pressure of 2 bar from a feed consisting of 12 mol of steam per mole of 1-butene.
(b) If the initial mixture consists of 50 mol% steam and 50 mol% 1-butene, how will the required temperature be affected?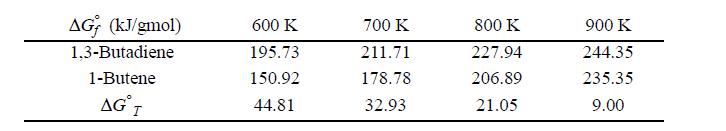
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Introductory Chemical Engineering Thermodynamics
ISBN: 9780136068549
2nd Edition
Authors: J. Elliott, Carl Lira
Question Posted:





