Consider problem 3.11 using benzene as the fluid rather than air and eliminating the ideal gas assumption.
Question:
Consider problem 3.11 using benzene as the fluid rather than air and eliminating the ideal gas assumption. Use the Peng-Robinson equation. For the same initial state,
(a) The final tank temperature will not be 499.6 K. What will the temperature be?
(b) What is the number of moles left in the tank at the end of the process?
(c) Write and simplify the energy balance for the process. Determine the final temperature of the piston/cylinder gas.
Data from problem 3.11
A well-insulated tank contains 1 mole of air at 2 MPa and 673 K. It is connected via a closed valve to an insulated piston/cylinder device that is initially empty. The piston may be assumed to be frictionless. The volumes of the piping and valve are negligible. The weight of the piston and atmospheric pressure are such that the total downward force can be balanced with gas pressure in the cylinder of 0.7 MPa. The valve between the tank and piston/cylinder is cracked open until the pressure is uniform throughout. The temperature in the tank is found to be 499.6 K. Air can be assumed to be an ideal gas with a temperatureindependent heat capacity CP = 29.3 J/mol-K.
What is the number of moles left in the tank at the end of the process?
Write and simplify the energy balance for the process. Determine the final temperature of the piston/cylinder gas.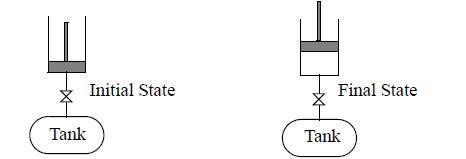
Step by Step Answer:

Introductory Chemical Engineering Thermodynamics
ISBN: 9780136068549
2nd Edition
Authors: J. Elliott, Carl Lira





