Sodium fluoride, NaF, is dissolved in water at an apparent concentration of C B = 10 -3
Question:
Sodium fluoride, NaF, is dissolved in water at an apparent concentration of CB = 10-3 mol/L. Construct a Sillèn diagram and estimate the pH. Refer to the pKa,A and pKa,B values in Table 18.2.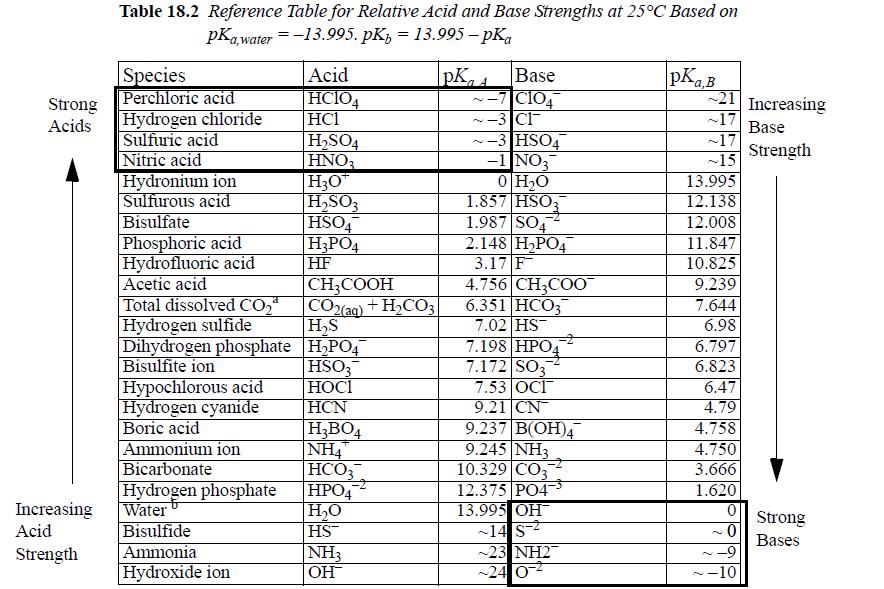
Transcribed Image Text:
Strong Acids Increasing Acid Strength Table 18.2 Reference Table for Relative Acid and Base Strengths at 25°C Based on pKa,water = -13.995. pKz 13.995-pka Species Perchloric acid Hydrogen chloride Sulfuric acid Nitric acid Hydronium ion Sulfurous acid Bisulfate Phosphoric acid Hydrofluoric acid Acetic acid Total dissolved CO₂ Hydrogen sulfide Dihydrogen phosphate Bisulfite ion Hypochlorous acid Hydrogen cyanide Boric acid Ammonium ion Bicarbonate Hydrogen phosphate Water Bisulfide Ammonia Hydroxide ion Acid HCIO4 HC1 H₂SO4 HNO3 H3O™ H₂SO3 HSO4 H3PO4 HF CH3COOH CO2(aq) + H₂CO3 H₂S H₂PO47 HSO3 НОСI HCN H₂BO4 |NH4 HCO3 HPO4 H₂O HS™ NH3 OH PKA Base ~-7 C104 ~-3 Cl -3 HSO4 -1 NO3 0 H₂O 1.857 HSO3 1.987 SO4 2.148 H₂PO4 3.17 F 4.756 CH3COO 6.351 HCO3 7.02 HS 7.198 HPO4 7.172 SO3 7.53 OCI 9.21 CN 9.237 B(OH)4 9.245 NH3 10.329 CO3 12.375 PO4³ 13.995 OH™ -14 S-2 ~23 NH2 -24 0² pKa,B 21 Increasing ~17 Base Strength ~17 ~15 13.995 12.138 12.008 11.847 10.825 9.239 7.644 6.98 6.797 6.823 6.47 4.79 4.758 4.750 3.666 1.620 0 ~0 -9 -10 N Strong Bases
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
Constructing a Silln diagram also known as a Bjerrum plot or a distribution diagram involves plotting the fraction of various species as a function of ...View the full answer

Answered By

Hemstone Ouma
"Hi there! My name is Hemstone Ouma and I am a computer scientist with a strong background in hands-on experience skills such as programming, sofware development and testing to name just a few. I have a degree in computer science from Dedan Kimathi University of Technology and a Masters degree from the University of Nairobi in Business Education. I have spent the past 6 years working in the field, gaining a wide range of skills and knowledge. In my current role as a programmer, I have had the opportunity to work on a variety of projects and have developed a strong understanding of several programming languages such as python, java, C++, C# and Javascript.
In addition to my professional experience, I also have a passion for teaching and helping others to learn. I have experience as a tutor, both in a formal setting and on a one-on-one basis, and have a proven track record of helping students to succeed. I believe that with the right guidance and support, anyone can learn and excel in computer science.
I am excited to bring my skills and experience to a new opportunity and am always looking for ways to make an impact and grow as a professional. I am confident that my hands-on experience as a computer scientist and tutor make me a strong candidate for any role and I am excited to see where my career will take me next.
5.00+
8+ Reviews
22+ Question Solved
Related Book For 

Introductory Chemical Engineering Thermodynamics
ISBN: 9780136068549
2nd Edition
Authors: J. Elliott, Carl Lira
Question Posted:
Students also viewed these Engineering questions
-
A large company is looking to acquire a smaller business. The company believes that, under their management, free cash flows for upcoming years should be forecasted as shown below, and would continue...
-
( ( i ) ) An abstract Use Case is one that is: A . . connected to an Actor but not initiated by another Use Case B . . connected to an Actor and initiated by another Use Case C . . not connected to...
-
Let Find all values of k for which: (a) A has eigenvalues 3 and -1. (b) A has an eigenvalue with algebraic multiplicity 2. (c) A has no real eigenvalues. k 2
-
What is the formula to find total dividend and payout ratio? This is the information I have: the amount of shares the company holds and the last dividend paid. Lastly, will there be enough cash to...
-
Explain the two types of problems and decisions. Contrast the three decision-making conditions.
-
Two rigid, insulated tanks are interconnected by a valve. Initially 0.79 kmol of nitrogen at 200 kPa and 255 K fills one tank. The other tank contains 0.21 kmol of oxygen at 100 kPa and 300 K. The...
-
Data extracted from a year-end balance sheet are shown below. Compute the working capital for this firm. What would the current ratio be, and what is the significance for this firm? Accounts payable...
-
Your client is 40 years old; and she wants to begin saving for retirement, with the first payment to come one year from now. She can save $5,000 per year; and you advise her to invest it in the stock...
-
24. Write the standard form of the equation of the circle with the given characteristics. Endpoints of a diameter: (4,3), (-14, -13) 25. Write the equation of the circle in standard form, (x-h)+(y-k)...
-
Calcium chloride is used occasionally as an alternative to sodium chloride for de-icing walkways. It is rumored to maintain puddles even a day or so after all evidence of sodium chloride has...
-
(a) Compute the freezing point depression for an aqueous solutions that is 3 wt% NaCl. (b) Compute the boiling point elevation for an aqueous solutions that is 3 wt% NaCl. (c) Compute the osmotic...
-
Iron in the earth is in the form of iron ore. Common ores include Fe 2 O 3 (hematite), Fe 3 O 4 (magnetite), and FeCO 3 (siderite). Calculate the mass percent composition of iron for each of these...
-
In downtown Chicago, the east-west blocks are 400 ft long while the north-south blocks are 280 ft long. Because of the many one-way streets, it can be challenging to get around. Veronica starts at...
-
The DuPont system allows us to break down the return on equity into: A. return on assets and the financial leverage ratio. B. profit margin, the tax retention ratio, and inventory turnover. C. gross...
-
Estimate the length of a human lifetime, in seconds.
-
The file collegetown contains data on 500 houses sold in Baton Rouge, LA during 2009-2013. Variable descriptions are in the file collegetown.def. a. Estimate the log-linear model \(\ln (P R I C...
-
Compare and contrast the terms discounting and compounding.
-
Paradise Burger Company makes two burgers, each in a separate division: cheeseburgers and chiliburgers. Segmented income statements for the most recent year are as follows: Paradise Burgers...
-
Create an appropriate display of the navel data collected in Exercise 25 of Section 3.1. Discuss any special properties of this distribution. Exercise 25 The navel ratio is defined to be a persons...
-
A 0.100 M solution of the weak acid HA was titrated with 0.100 M NaOH. The pH measured when V b = V e was 4.62. Using activity coefficients, calculate pKa. The size of the A - anion is 450 pm.
-
Finding the end point from pH measurements. Here are data points around the second apparent end point in Figure 10-5: (a) Prepare a spreadsheet or table analogous to Figure 10-6, showing the first...
-
Indicator error. Consider the titration in Figure 10-2 in which the equivalence-point pH in Table 10-2 is 9.25 at a volume of 10.00 mL. (a) Suppose you used the yellow-to-blue transition of thymol...
-
Research how multinational companies like the Fortune 500 companies can use the process of Scenario Planning to determine how they have and can adapt to an uncertain world that includes COVID-19, the...
-
The average total compensation (includes tips, bonus, and overtime pay) for the entry-level Insurance Agent with less than 5 years' experience in a sample of 100 agents across Ontario shows the...
-
http://www.nbcnews.com/id/23300489/ns/business-us_business/t/do-you-want-fries-zen/#.V6OmL01-O70 Please read the article and briefly describe what McDonalds did. Do you think such a practive will...

Study smarter with the SolutionInn App


