Sometimes we would like to enhance the solubility of a drug by adding a cosolvent, instead of
Question:
Sometimes we would like to enhance the solubility of a drug by adding a cosolvent, instead of adding antisolvent to precipitate. Making optimal use of the data in the previous problem, estimate the amount of ethanol that should be added to water to prepare an aqueous solution of salicylic acid with concentration of 10 wt% at 25°C.
Data from Problem 35:
Salicylic acid is similar in structure to aspirin. Shalmashi et al. have measured the data in Table 14.5.
(a) Find the value of α for salicylic acid in water that best correlates the data, assuming β = 0.
(b) Predict the solubility of the acid in ethanol.
(c) Plot log(xacid) versus 1000/T including correlated and measured values.
(d) Plot all the calculated versus experimental values. This is known as a parity plot.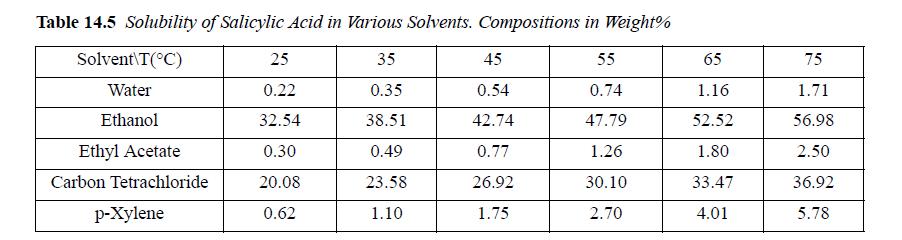
Step by Step Answer:

Introductory Chemical Engineering Thermodynamics
ISBN: 9780136068549
2nd Edition
Authors: J. Elliott, Carl Lira





