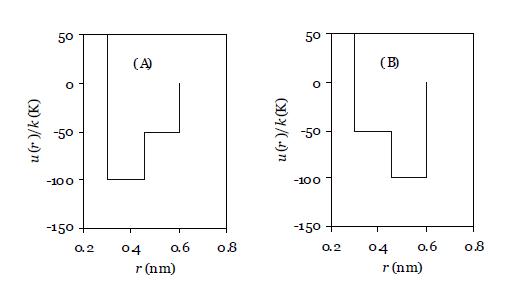
Suppose two molecules had similar potential functions, but they were mirror images of one another as shown
Question:
Suppose two molecules had similar potential functions, but they were mirror images of one another as shown in the figure below. Which one (A or B) would have the larger internal energy departure? You may assume that the radial distribution function is the same for both potential models.
(a) Reason qualitatively but refer to appropriate equations to explain your answer.
(b) Compute the values of (U – Uig)/RT at a packing fraction of 0.4 and a temperature of 50 K. Assume values of the radial distribution function as follows: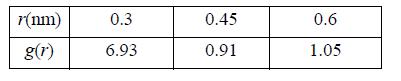
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Introductory Chemical Engineering Thermodynamics
ISBN: 9780136068549
2nd Edition
Authors: J. Elliott, Carl Lira
Question Posted:





