The vapor-liquid equilibria for the system acetic acid(1) + acetone(2) needs to be characterized in order to
Question:
The vapor-liquid equilibria for the system acetic acid(1) + acetone(2) needs to be characterized in order to simulate an acetic anhydride production process. Experimental data for this system at 760 mmHg have been reported by Othmer (1943)4 as summarized below. Use the data at the equimolar composition to determine a value for the binary interaction parameter of the Peng-Robinson equation. Based on the value you determine for the binary interaction parameter, determine the percent errors in the Peng-Robinson prediction for this system at a mole fraction of x(1) + 0.3.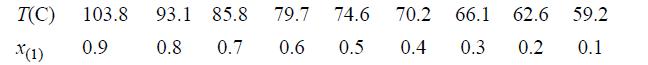
Transcribed Image Text:
T(C) 103.8 X(1) 0.9 93.1 85.8 79.7 74.6 0.8 0.7 0.6 0.5 70.2 66.1 62.6 59.2 0.4 0.3 0.2 0.1
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (3 reviews)

Answered By

Saikumar Ramagiri
Financial accounting:- Journal and ledgers, preparation of trail balance and adjusted trail balance Preparation of income statement, retained earning statement and balance sheet Banks reconciliation statements Financial statement analysis Cash flow statement analysis (both direct and indirect methods) All methods of Depreciations Management Accounting:- Ratios Budgeting control Cash budget and production budget Working capital management Receivable management Costing:- Standard and variance costing Marginal costing and decision making Cost-volume-profit analysis Inventory management (LIFO, FIFO) Preparation and estimation of cost sheet Portfolio management:- Calculation of portfolio standard deviation or risk Calculation of portfolio expected returns CAPM, Beta Financial management:- Time value of money Capital budgeting Cost of capital Leverage analysis and capital structure policies Dividend policy Bond value calculations like YTM, current yield etc International finance:- Derivatives Futures and options Swaps and forwards Business problems Finance problems Education (mention all your degrees, year awarded, Institute/University, field(s) of major): Education Qualification Board/Institution/ University Month/Year of Passing % Secured OPTIONALS/ Major ICWAI(inter) ICWAI inter Pursuing Pursuing - M.com(Finance) Osmania University June 2007 65 Finance & Taxation M B A (Finance) Osmania University Dec 2004 66 Finance & Marketing. B.Com Osmania University June 2002 72 Income Tax, Cost & Mgt, Accountancy, Auditing. Intermediate (XII) Board of Intermediate May 1999 58 Mathematics, Accountancy, Economics. S S C (X) S S C Board. May 1997 74 Mathematics, Social Studies, Science. Tutoring experience: • 10 year experience in online trouble shooting problems related to finance/accountancy. • Since 6 Years working with solution inn as a tutor, I have solved thousands of questions, quick and accuracy Skills (optional): Technical Exposure: MS Office, SQL, Tally, Wings, Focus, Programming with C Financial : Portfolio/Financial Management, Ratio Analysis, Capital Budgeting Stock Valuation & Dividend Policy, Bond Valuations Individual Skills : Proactive Nature, Self Motivative, Clear thought process, Quick problem solving skills, flexible to complex situations. Achievements : 1. I have received an Award certificate from Local Area MLA for the cause of getting 100% marks in Accountancy during my Graduation. 2. I have received a GOLD MEDAL/Scholarship from Home Minister in my MBA for being the “Top Rank student “ of management institute. 3. I received numerous complements and extra pay from various students for trouble shooting their online problems. Other interests/Hobbies (optional): ? Web Surfing ? Sports ? Watching Comics, News channels ? Miniature Collection ? Exploring hidden facts ? Solving riddles and puzzles
4.80+
391+ Reviews
552+ Question Solved
Related Book For 

Introductory Chemical Engineering Thermodynamics
ISBN: 9780136068549
2nd Edition
Authors: J. Elliott, Carl Lira
Question Posted:
Students also viewed these Engineering questions
-
Looking at the data about contacting mom (exercise 13.49), for which group (ethicists or other) could you perform a log transform, and for which group could you not? Explain. Exercise 13.49 Eth Other...
-
A bakery wants to determine how many trays of doughnuts it should prepare each day. Demand is normal with a mean of 5 trays and standard deviation of 2 trays. If the owner wants a service level of at...
-
The Moondance Riding Academy held its annual horse show for 3 days. The total amount collected in entry fees for the 3 days was $1,450. The amount collected, in dollars, is shown for each of the 3...
-
The table represents values of differentiable functions f and g and their first derivatives. Use the table of values to answer the questions that follow. Work all of the parts below the line. X f g...
-
Bertha Jamison contracted to purchase a set of encyclopedias from Encyclopedia Britannica for $1,652.08. She made a $100 down payment and signed a document entitled "Britannica Revolving Credit...
-
The amount of red meat consumed in the United States has remained fairly constant in recent years while the amount of poultry consumed has increased during those years, as shown by the data in the...
-
Use the implicit finite difference method to solve the heat conduction problem on the unit square: \[\begin{aligned}& \frac{\partial^{2} u}{\partial x^{2}}=\frac{\partial u}{\partial t} \\& u(x, 0)=x...
-
A company builds custom equipment, [t has landed a contract with a major customer. Relevant data are shown below. The complication is that the delivery has been promised in 32 weeks and the company...
-
(i) f(x)=x-5 (ii) f(x) Examine the following functions for continuity. 1 X-5 = 5x5 x-25 (iii) f(x) = x-5 (iv) f(x) = |x-5| x + 5
-
A gaseous mixture of 30 mol% CO 2 and 70 mol% CH 4 enters a valve at 70 bar and 40C and exits at 5.3 bar. Does any CO 2 condense? Assume that the mixture follows the virial equation. Assume that any...
-
The procedure for calculation of the residual enthalpy for a pure gas is shown in Example 8.5. Now consider the residual enthalpy for a binary gas mixture. For this calculation, it is necessary to...
-
A railroad flatcar is traveling to the right at a speed of 13.0 m/s relative to an observer standing on the ground. Someone is riding a motor scooter on the flatcar (Fig. 3.43). What is the velocity...
-
An mRNA encodes a polypeptide that is 312 amino acids long. The 53rd codon in this polypeptide is a tryptophan codon. A mutation in the gene that encodes this polypeptide changes this tryptophan...
-
The total amount of G plus C in an organisms DNA is 64% of the total base content of that DNA. What are the percentages of A, T, G, and C in the DNA?
-
The trait of feathering in fowls is a sex-limited trait controlled by a single gene. Females always exhibit hen-feathering, as do HH and Hh males. Only hh males show cock-feathering. Starting with...
-
Within a protein, certain amino acids are positively charged (e.g., lysine and arginine), some are negatively charged (e.g., glutamate and aspartate), some are polar but uncharged, and some are...
-
Lactose permease, a protein produced in E. coli, is composed of a single polypeptide that is 417 amino acids long. By convention, the amino acids within a polypeptide are numbered from the...
-
Whitney R.V.s Inc. has invested its excess cash in the following instruments during December 2008: Certificate of deposit, due Jan. 31, 2009 .......... $100,000 Certificate of deposit, due May 31,...
-
Factor and simplify, if possible. Check your result using a graphing calculator. 3 cot 2 + 6 cot + 3
-
Go to Google Patents and find a patent for an innovative mechanical device that you think would not be a success in the marketplace. Since it is patented, it is considered innovative. Therefore,...
-
For the magnesium camera body shown, provide an explanation for which processes you think were used in its manufacture and why. See Figure P2.27. Figure P2.27.
-
For the aluminum structural member shown, provide an explanation for which processes you think were used in its manufacture and why. See Figure P2.28. Figure P2.28.
-
How can we formulate actionable strategies that leverage predictive analytics to anticipate market trends and consumer behavior shifts ?
-
How do you calculate a project's estimated net cash flow stream and what is the significance of it?
-
Explain why personal bankruptcy should be the choice of last resort.

Study smarter with the SolutionInn App


