Two moles of nitrogen are initially at 10 bar and 600 K (state 1) in a horizontal
Question:
Two moles of nitrogen are initially at 10 bar and 600 K (state 1) in a horizontal piston/cylinder device. They are expanded adiabatically to 1 bar (state 2). They are then heated at constant volume to 600 K (state 3). Finally, they are isothermally returned to state 1. Assume that N2 is an ideal gas with a constant heat capacity as given on the back flap of the book. Neglect the heat capacity of the piston/cylinder device. Suppose that heat can be supplied or rejected as illustrated below. Assume each step of the process is reversible.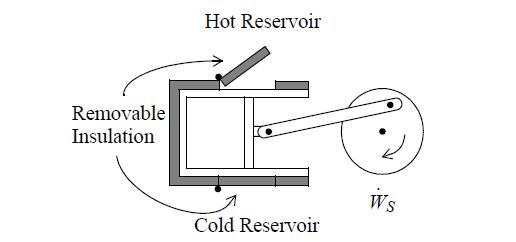
(a) Calculate the heat transfer and work done on the gas for each step and overall.
(b) Taking state 1 as the reference state, and setting URig = 0, calculate U and H for the nitrogen at each state, and ΔU and ΔH for each step and the overall Q and WEC.
(c) The atmosphere is at 1 bar and 298 K throughout the process. Calculate the work done on the atmosphere for each step and overall.
Step by Step Answer:

Introductory Chemical Engineering Thermodynamics
ISBN: 9780136068549
2nd Edition
Authors: J. Elliott, Carl Lira





