Use Eq. (13.13) to reduce one of the following isothermal data sets, and compare the result with
Question:
Use Eq. (13.13) to reduce one of the following isothermal data sets, and compare the result with that obtained by application of Eq. (13.19). Recall that reduction means developing a numerical expression for GE∕RT as a function of composition.
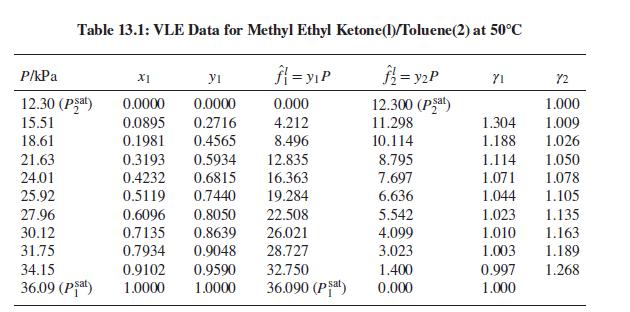
(a) Methylethylketone(1)/toluene(2) at 50°C: Table 13.1.
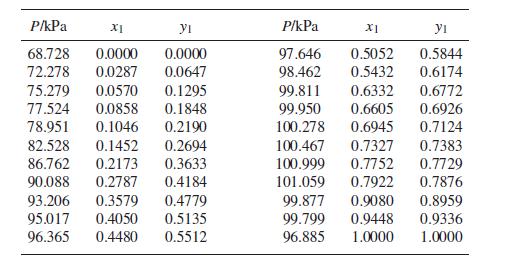
(b) Acetone(1)/methanol(2) at 55°C: Prob. 13.34.
(c) Methyl tert-butyl ether(1)/dichloromethane(2) at 35°C: Prob. 13.37.
(d) Acetonitrile(1)/benzene(2) at 45°C: Prob. 13.40.
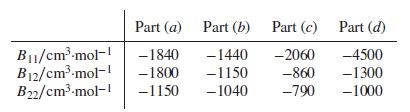
Second-virial-coefficient data are as follows:
Eq. (13.13)
![]()
Eq. (13.19)
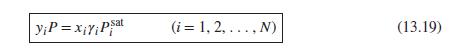
(a) Table 13.1
(b) Prob. 13.34
The following is a set of VLE data for the system acetone(1)/methanol(2) at 55°C:
(c) Prob. 13.37
VLE data for methyl tert-butyl ether(1)/dichloromethane(2) at 308.15 K are as follows:
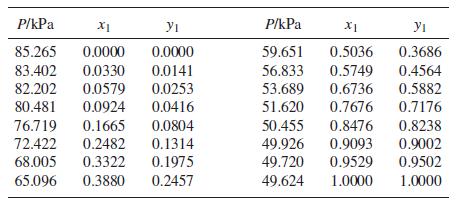
(d) Prob. 13.40
Following are VLE data for the system acetonitrile(1)/benzene(2) at 45°C:
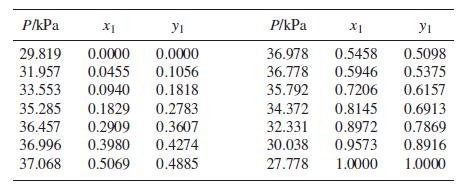
Step by Step Answer:

Introduction To Chemical Engineering Thermodynamics
ISBN: 9781259696527
8th Edition
Authors: J.M. Smith, Hendrick Van Ness, Michael Abbott, Mark Swihart





