To determine how well existing chemical analyses can detect lead in test specimens in water, a civil
Question:
To determine how well existing chemical analyses can detect lead in test specimens in water, a civil engineer submits specimens spiked with known concentrations of lead to a laboratory. The chemists are told only that all samples are from a study about measurements on "low" concentrations, but they are not told the range of values to expect. This is sometimes called a calibration problem because the goal is to relate the measured concentration \((y)\) to the known concentration \((x)\). Given the data (Courtesy of Paul Berthouex)
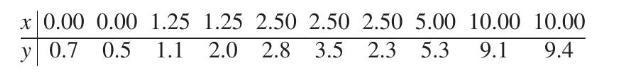
(a) plot measured concentration versus known concentration; comment on the pattern;
(b) fit a straight line by least squares;
(c) if the chemical test is correct, on average, we would expect a straight line that has slope 1 . Obtain a \(95 \%\) confidence interval for \(\beta\);
(d) test \(H_{0}: \beta=1\) versus \(H_{1}: \beta eq 1\) at level \(\alpha=0.05\).
Step by Step Answer:

Probability And Statistics For Engineers
ISBN: 9780134435688
9th Global Edition
Authors: Richard Johnson, Irwin Miller, John Freund




