A rigid, well-insulated container is initially divided into three compartments. The top compartment contains a vacuum. It
Question:
A rigid, well-insulated container is initially divided into three compartments. The top compartment contains a vacuum. It is separated from the middle compartment A by a frictionless mass of 1000 kg and area 0.098 m2. Compartment A contains 2 moles of ideal gas at 300 K and is separated from compartment B, on the bottom of the container, by a rigid partition. Compartment B initially contains 2 moles of the same ideal gas at 300 K and occupies a volume of 0.1 m3 A process is initiated by removing the partition. The mass then re-equilibrates in the container. What is the change in entropy? Take cP = (5/2)R.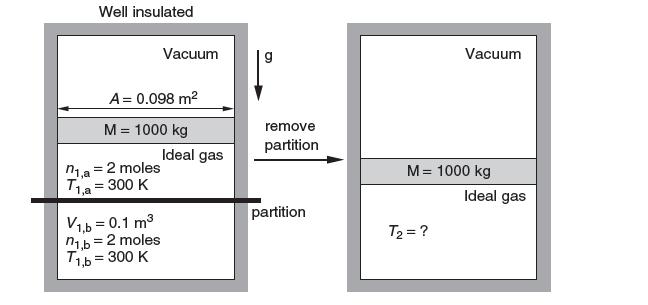
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





