Consider a binary liquid mixture of hexane (1) and acetone (2). At 15C and 300 bar, this
Question:
Consider a binary liquid mixture of hexane (1) and acetone (2). At 15°C and 300 bar, this mixture forms two partially miscible liquid phases. Phase α has 20 total moles with x1α = 0.2, while phase b has 10 total moles with x1β = 0.8. The following data are available at 15°C: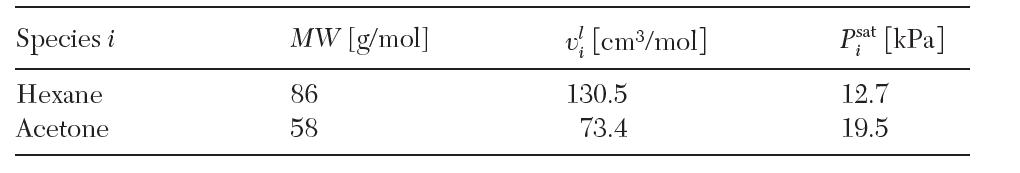
(a) Draw a schematic of the system, labeling it with all the information that you have. Make your schematic as accurate as possible; for example, consider which phase belongs on top.
(b) Are the like interactions stronger or weaker than the unlike interactions? Explain.
(c) Calculate the value of f1.
(d) You wish to use the two-suffi x Margules equation to describe this system. Based on the data above, come up with a value for the two-suffi x Margules parameter, A.
(e) Estimate to what temperature you need to bring the system described above to make it completely miscible, that is, to make it have only one phase present. State the important assumptions that you make.
(f) Estimate the value of H1 at 15°C and 300 bar.
Step by Step Answer:





