Consider a high-pressure tank at room temperature. It undergoes a process where a valve is opened and
Question:
Consider a high-pressure tank at room temperature. It undergoes a process where a valve is opened and the gas escapes until the pressure reaches 1 bar.
(a) The process is undertaken with an ideal gas, as shown as system A. Will the fi nal temperature T2A be greater than, equal to, or less than 298 K? Explain.
(b) Consider now a tank of propane, a real gas, at the same initial pressure as the tank in part A. This tank undergoes an identical process where it is opened and the gas escapes until it too reaches a pressure of 1 bar. It obtains a fi nal temperature T2B. This is shown as system B. Will the fi nal temperature T2B be greater than, equal to or less than T2A? Explain.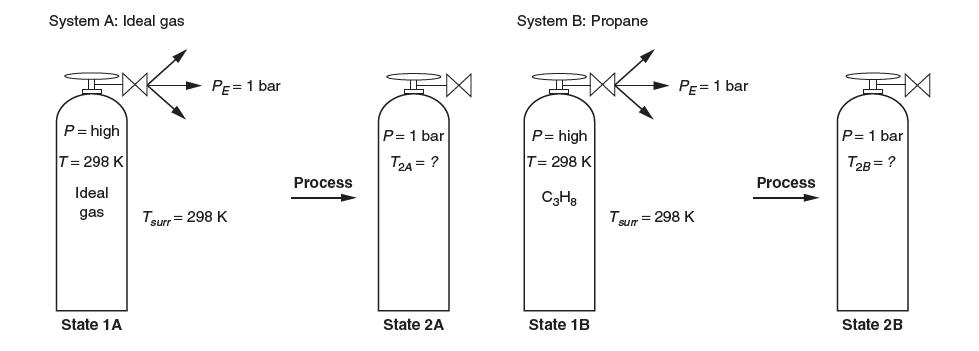
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





