Consider a mixture of CH4 and H2S at 444 K and 70 bar. Use the results of
Question:
Consider a mixture of CH4 and H2S at 444 K and 70 bar. Use the results of Example 7.4 to plot the fugacity coeffi cient of methane as a function of methane mole fraction using the van der Waals equation of state and mixing rules. Compare the result with that obtained from the text software, ThermoSolver, using the Peng–Robinson equation of state.
EXAMPLE 7.4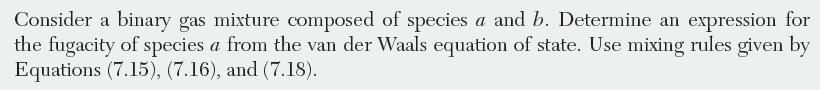
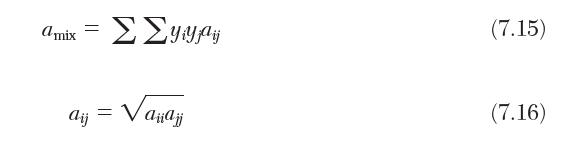

Transcribed Image Text:
Consider a binary gas mixture composed of species a and b. Determine an expression for the fugacity of species a from the van der Waals equation of state. Use mixing rules given by Equations (7.15), (7.16), and (7.18).
Step by Step Answer:

This question has not been answered yet.
You can Ask your question!
Related Book For 

Question Posted:
Students also viewed these Engineering questions
-
Consider a mixture of CH4 and H2S at 444 K and 70 bar. Calculate the fugacity coeffi cient of methane in an equimolar mixture using each of the following: (a) the van der Waals equation of state (b)...
-
Van der Waals Equation and Critical Points (a) In p V- diagrams the slope p/V along an isotherm is never positive. Explain why. (b) Regions where p/V = 0 represent equilibrium between two phases;...
-
The overall goal of this problem is to compute the PV and PT equilibrium diagramsfor a single component fluid described by the van derWaals equation of state. Let us recall the key things we need to...
-
Saeed does not keep proper books of account for his business but he has provided the following details of his assets and liabilities. Further information 1. Land and buildings have been revalued at...
-
The following information has been taken from the financial statements for Payne plc (Payne) for the year ended 31 March 2013. *Statement of Profit or Loss and Other Comprehensive Income (extracts)...
-
AmeriPlas, Inc., produces 20-ounce plastic drinking cups that are embossed with the names of prominent beers and soft drinks. The sales data are: Date Sales Jan-13 40,358 Feb-13 45,002 Mar-13 63,165...
-
Part of the required input to the viscoelastic option in some finite element codes is a table showing the time-dependent, isotropic shear modulus \(G(t)\) at different times \(t\). Explain how you...
-
You have been asked to advise a business-to-business manufacturing company how to detect fraudulent financial reporting. Management does not understand how early revenue recognition by backdating...
-
What are the two most interesting guidelines that relate to a strategic planning team formulating a vision of success? Why did you find these two interesting? What are the desired outcomes of an...
-
You wish to represent a binary mixture of species a and b at 127C and 80 bar by the virial equation. At 127C, the second virial coeffi cients are given by: You have also found that at infi nite...
-
The following form of a cubic equation of state has been recommended by Martin: P = RT v-b a (v + c)
-
On graduation day at a large university, one graduate is selected at random. Let A represent the event that the student is an engineering major, and let B represent the event that the student took a...
-
Describe the objectives and operations of defensive and offensive operations. Describe the operations performed during a transitional attack. Describe the characteristics of a solid stream, straight...
-
Under current law, establishing a union is a 2 step process. First a certain number of signature cards are needed for the NLRB to hold a secret ballot election to determine if the majority of the...
-
describe systems and processes which facilitate continuous improvement within organizations. Discuss the relevance of each system and process to continuous improvement. System E.g., Customer...
-
discusses drug testing extensively (and approvingly). Not mentioned is that currently twenty states have legalized recreational marijuana. Another sixteen allow its medical use. How might you...
-
In a situation where employee relations are poor, powers with manager are high and task structure is high, which Leadership style should be chosen?
-
Are these marketing activities appropriate for this product? Meet us before you need usthats the motto of a cemetery in Denver. Facing decreasing demand as more Americans choose cremation, cemeteries...
-
During 2012, Cheng Book Store paid $483,000 for land and built a store in Georgetown. Prior to construction, the city of Georgetown charged Cheng $1,300 for a building permit, which Cheng paid. Cheng...
-
I-Butanol and chlorobenzene form a minimum-boiling azeotropic system. The mole fraction of I-butanol in the liquid (x) and vapour (y) phases at 1.000 atm is given below for a variety of boiling...
-
The following data have been obtained for the liquid-vapour equilibrium compositions of mixtures of nitrogen and oxygen at 100 kPa. T/K 77.3 78 80 82 84 86 88 90.2 X (O2) 0 10 34 54 70 82 92 100...
-
The table below gives the break and halt temperatures found in the cooling curves of two metals A and B. Construct a phase diagram consistent with the data of these curves. Label the regions of the...
-
Indigo Inc.'s $11 par value common stock is actively traded at a market price of $15 per share. Indigo issues 6,500 shares to purchase land advertised for sale at $85,500. Journalize the issuance of...
-
you want to have 10,000 seven years from now how much will you have to save each month if the annual interest is 6% compounded monthly
-
Radisson Square Garden Entertainment (RSGM) reported net income of $100 million in 2022, after interest expenses of $10 million. (The corporate tax rate was 20%.) It reported depreciation of $15...

Study smarter with the SolutionInn App


