Consider a process whereby a crystal of silicon is placed in a furnace with diborane gas, B2H6.
Question:
Consider a process whereby a crystal of silicon is placed in a furnace with diborane gas, B2H6. Using the concepts of defect equilibria, we wish to understand the effect of diborane pressure in the furnace on doping concentration. Consider the following reactions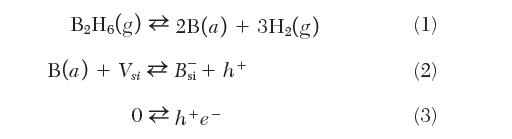
where B
(a) is adsorbed boron on the silicon surface.
(a) Write equilibrium constant expressions for the three reactions above.
(b) Based on the species described above, write the condition for charge neutrality (electroneutrality).
(c) Consider the furnace at constant temperature. In this case, the concentration of silicon vacancies is a constant. Consider two regions possible—low diborane partial pressure and high diborane partial pressure. Come up with a qualitative plot of log[p] and log[n] vs. log pB2H6 and indicate each region.
Step by Step Answer:





