Consider the industrial production of cyclohexane, C6H12, by the gas-phase hydrogenation of benzene, C6H6. Assume that this
Question:
Consider the industrial production of cyclohexane, C6H12, by the gas-phase hydrogenation of benzene, C6H6. Assume that this process is carried out by two reactors in series as shown below.
The fi rst reactor is at 340°C and 5 bar, while the second reactor is at 265°C and 5 bar. The feed ratio of hydrogen gas to benzene is 10:1; there is no cyclohexane in the feed. All species are in the gas phase. You may assume ideal gas behavior and that Δhrxn o does not change with temperature.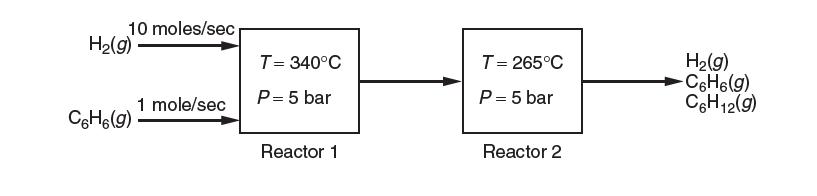
(a) What is the equilibrium composition at the exit of the second reactor?
(b) What is the purpose of the fi rst reactor; that is, why do we use two reactors instead of just one?
(c) Would we get more product if we used a pressure of 1 bar instead of 5 bar. Explain.
(d) Would you recommend diluting the feed with an inert to increase the yield of C6H12? Explain.
Step by Step Answer:






