Develop a general relationship for the change in temperature with respect to pressure at constant entropy: (a)
Question:
Develop a general relationship for the change in temperature with respect to pressure at constant entropy: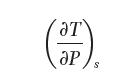
(a) Evaluate the expression for an ideal gas.
(b) From the result in part (a), show that for an ideal gas with constant cP, an isentropic expansion from state 1 and state 2 yields Equation (2.49).
(c) Evaluate the expression for a gas that obeys the van der Waals equation of state.![]()
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





