Example 8.6 illustrates how you solve a dew-point calculation for a binary mixture of a nonideal liquid
Question:
Example 8.6 illustrates how you solve a dew-point calculation for a binary mixture of a nonideal liquid and a nonideal gas with T known. This problem corresponds to quadrant I in Figure 8.2.
Develop an analogous solution for the bubble point with the liquid-phase mole fractions and T known (quadrant II). As in Example 8.6, use the van der Waals equation for vapor nonideality and the three-suffi x Margules equation for liquids. Assume that critical properties, liquid volumes, and Antoine coeffi cients for each species are readily available and that the three-suffi x Margules parameters have been determined.
Example 8.6
At high pressures, both the vapor and liquid phases may be nonideal. Consider a binary mixture of a and b with vapor-phase mole fraction and T known. Develop a set of equations and a solution algorithm to determine the composition in the liquid phase and the system pressure.
Use the van der Waals equation to quantify deviations from ideality in the vapor and the threesuffi x Margules equation to model the nonideal liquid. Assume that critical properties, liquid volumes, and Antoine coeffi cients for each species are readily available and that the threesuffi x Margules parameters have been determined.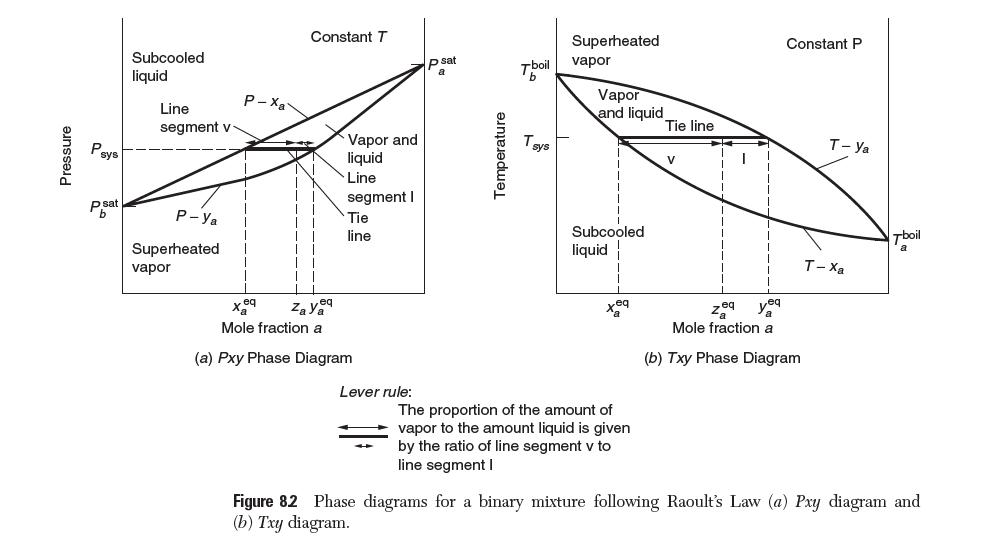
Step by Step Answer:






