One step in the manufacture of solar cells is the growth of solid silicon from gas feed.
Question:
One step in the manufacture of solar cells is the growth of solid silicon from gas feed. Consider a feed source of pure chlorosilane that undergoes the following reaction: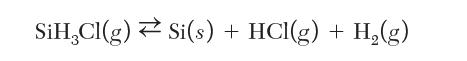
You are considering a process that runs at 500°C and 100 Pa. Answer the following questions.
(a) What is the maximum amount of Si product you can make for each mole of SiClH3 fed into the reactor? You may assume Δh° rxn = const.
(b) What data would you need to calculate an answer without assuming Δh° rxn = const? How would that change your calculation?
(c) Based on your answers to part A and B, how reasonable do you think the assumption that Δh° rxn = const is? Given time and data, should you recalculate your answer?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





