phase diagram for the solid liquid equilibrium of a binary mixture of silver (Ag) and copper (Cu)
Question:
phase diagram for the solid liquid equilibrium of a binary mixture of silver (Ag) and copper (Cu) is shown below. Answer the following questions. Note that the weight percentage is on the bottom and the mole percentage is on the top.
(a) What is the lowest temperature at which a binary mixture can exist entirely in the liquid phase?
What is its composition?
(b) What is the most copper that can be present in a phase of solid silver? At what temperature does this occur?
(c) Consider a liquid mixture of 1 mole Ag and 4 moles Cu at 800°C. At equilibrium, what phases exist and what are their compositions? How many moles are present in each phase?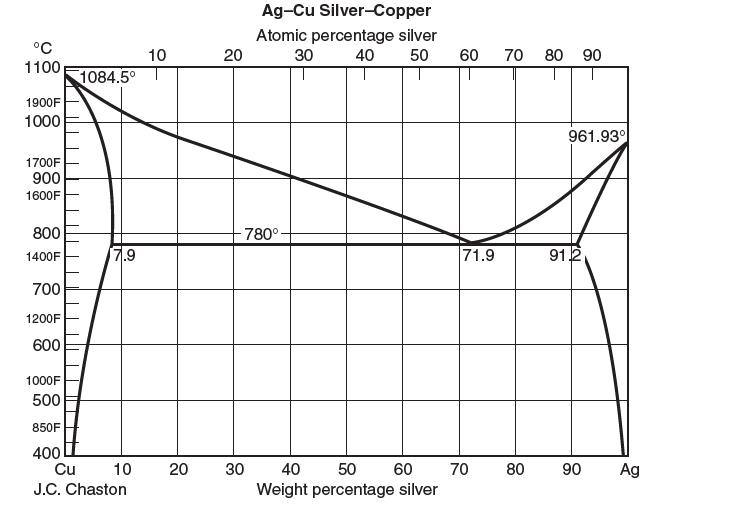
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





