The following expression describes the relation between pressure and molar volume of pure SF6 vapor at 30C,
Question:
The following expression describes the relation between pressure and molar volume of pure
SF6 vapor at 30°C, where P is in bar and v is in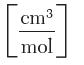
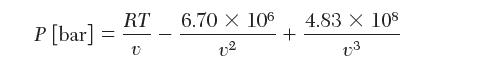
Consider a container that is 10 L (10,000 cm3) that contains 10.79 mol of SF6 vapor at 30°C.
Answer the following.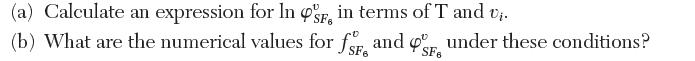
Transcribed Image Text:
cm mol 3 C
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)

Answered By

Rodrigo Louie Rey
I started tutoring in college and have been doing it for about eight years now. I enjoy it because I love to help others learn and expand their understanding of the world. I thoroughly enjoy the "ah-ha" moments that my students have. Interests I enjoy hiking, kayaking, and spending time with my family and friends. Ideal Study Location I prefer to tutor in a quiet place so that my students can focus on what they are learning.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Engineering questions
-
help ! i cant get this answer ! Suppose the iengths of human pregnancies ace nomally diesnibuted with \( \mu=266 \) days and o \( =16 \) eyss. Camplete part \( (a) \) and (b) below. (a) The figure to...
-
Pressure is related to volume by the relationship PV1.2 = Const, where P is in kPa and V is in m3. Determine the work required to compress an ideal gas from 0.8 m3 to 4000 cm3 if the initial pressure...
-
The AB Steel Plant The Vice President for Production at the AB Steel Plant was giving the Production Department Manager, Mr. Singh, a hard time for not doing anything about his work group which was...
-
Ashby and Curtis, married professionals, have a 2-year-old son, Jason. Curtis works full-time as an electrical engineer, but Ashby has not worked outside the home since Jason was born. As Jason is...
-
Read Bradley & Mahmood, "Bombardier TEG" case and Summarize the salient changes in his market and competition during the time of the case; Critique the "threat" of Morrison Knudsen; Perform a...
-
1. Is the new test a logical outgrowth of the original test? Why or why not? 2. How could the IRS have avoided a successful challenge to this new rule? The Internal Revenue Service (IRS) proposed a...
-
The plate in Figure 7.70 has edge dimensions \(a\) and \(b\) and is made from a \([90 / 0 / 90]_{s}\) symmetric cross-ply laminate. The plate is simply supported on all edges and is subjected to a...
-
Helner Cell Phones (HCP) is developing a new touch screen smartphone to compete in the cellular phone industry. The phones will be sold at wholesale prices to cell phone companies, which will in turn...
-
addi x2, x0, 16 slli x2, x2, 4 What is the value of x2 in decimal after the execution?
-
You have an unknown pure gas. Its volume has been measured to be 1.86 * 10-3 [m3/mol] at 15 bar and 100C and 6.12 * 104 [m3/mol] at 40 bar and 100C. As best you can, calculate the fugacity and...
-
In 1920, Schrieber proposed the following equation of state: where k, b, and c are constants. Develop an expression for the pure species fugacity coeffi cient of species i, ln vi , based on the...
-
In Exercises 2762, graph the solution set of each system of inequalities or indicate that the system has no solution. [x + y < 16 y = 2x IV
-
Describe a case where a retail company successfully implemented a loyalty program. How did this impact their customer retention and overall sales?
-
The following table represents the data for an economy in 2017 Category Number (Millions) Labor Force 420 Employed workers 395 Working age population 450 Population 500 Frictional Unemployment 1%...
-
Describe sources of information or data you can analyze and how to use these to review and improve your performance?
-
Describe the definition of the measure. Explain the numerical description of how the measure is constructed (the numerator/denominator measure counts, the formula used to construct the rate, etc.)....
-
Describe all about design of product and services in supply chain and management?
-
Using the full spectrum of segmentation variables, describe how Starbucks initially segmented and targeted the coffee market. By now, you should be familiar with the Starbucks story. After a trip to...
-
Organizations are increasing their use of personality tests to screen job applicants. What are some of the advantages and disadvantages of this approach? What can managers do to avoid some of the...
-
A laser used to read CDs emits red light of wavelength 700 nm. How many photons does it emit each second if its power is? (a) 0.10 W, (b) LOW?
-
The work function for metallic cesium is 2.14 eV. Calculate the kinetic energy and the speed of the electrons ejected by light of wavelength (a) 700 nm, (b) 300 nm.
-
Calculate the size of the quantum involved in the excitation of (a) An electronic oscillation of period 2.50 fs, (b) A molecular vibration of period 2.21 fs, (c) A balance wheel of period 1.0 ms....
-
Determine the steps of a personal development plan, below, to improve your ability to work with others. Below are examples to assist in the thought process of your needs Examples What do you do that...
-
Tax Table Not over Tax Rate Taxable income over O S 50,000 25 % 75.000 50,000 75,000 100.000 335,000 34 39 % 34 % 35 % 100,000 10,000,000 335,000 10,000,000 15,000,000 18,333,333 38 % 15,000,000 35 %...
-
Easy credit inc reported cash sales of $45000 credit sales of $280000 and write offs of bad debts of $7000 for last year. Accounts receivables had a balance of $327000 at the beginning of the year...

Study smarter with the SolutionInn App


