The following values (in kcal/mol) are reported for the Gibbs energy of formation of isomers of C9H20
Question:
The following values (in kcal/mol) are reported for the Gibbs energy of formation of isomers of C9H20 at 1000 K 14. We wish to calculate the equilibrium composition at 1000 K and 1 atm.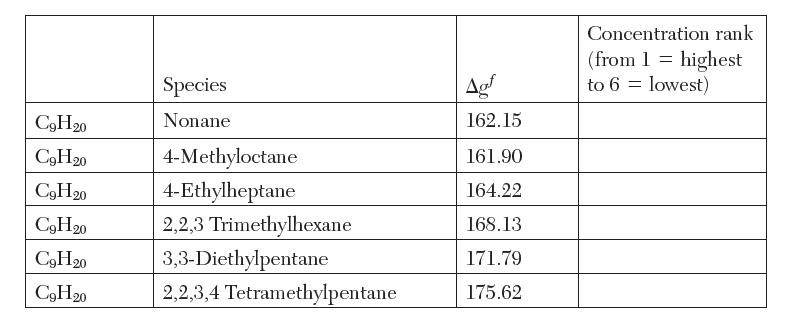
(a) By inspection, we can estimate what are the important species to consider. On the table, put these gas phase species in order from highest expected concentration to lowest concentration.
(b) Considering only the three species with the greatest concentrations, calculate the equilibrium composition, in mole fraction, of the mixture.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





