The standard half-cell potential for the reduction of water to form hydroxyl ion in Table 9.1 is
Question:
The standard half-cell potential for the reduction of water to form hydroxyl ion in Table 9.1 is reported as -0.828 V. If instead we write the reaction: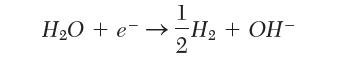
what should we use for the half-cell potential?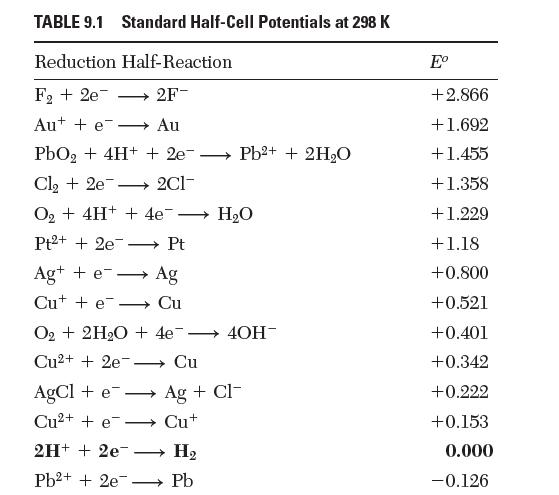
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





