You have been tasked with the removal of H2S and SO2 from an industrial stack. The following
Question:
You have been tasked with the removal of H2S and SO2 from an industrial stack. The following reaction is proposed to dispose of both species at once:![]()
Consider this reaction at 500°C. PvT data for H2S and SO2 have been fi t to the following equation of state: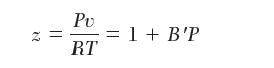
with values of B′ - 2.2 * 10-9 and -4.4 * 10-9 [Pa-1] for pure H2S and pure SO2, respectively. For H2O, use the steam tables for thermodynamic properties. For simplicity, you may use the following equation for the variation of the enthalpy of reaction with temperature: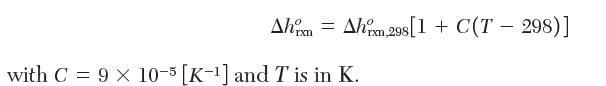
(a) Find an expression for the pure species fugacity coeffi cients of H2S and SO2 as a function of pressure at 500°C. P should be the only variable in your fi nal expression. Solve for w explicitly.
(b) Calculate the equilibrium constant at 500°C.
(c) To see if this reaction scheme is plausible, a high-pressure laboratory reactor is set up using an inlet stream of only H2S and SO2, consisting of 75% H2S and 25% SO2. What is the equilibrium conversion, j, at 10 MPa? You may approximate the fugacity coeffi cients in the mixture by their pure species fugacity coeffi cients.
(d) If a higher conversion is desired than that calculated in part (c), would you increase or decrease P? Explain.
(e) Quickly and roughly estimate the approximate pressure needed to obtain ξ = 0.95 at 500°C.
(f) Another possible way to obtain higher conversion is to change T. Would you increase or decrease T? Explain. In a real reactor, why would it be better to change P than T?
(g) There are many other species that do not take part in the reaction above. Would the addition of inerts lead to a greater conversion than that calculated in part (d)? Explain.
Step by Step Answer:






