You wish to fi t the benzene (1)isooctane (2) system to the following model for gE: The
Question:
You wish to fi t the benzene (1)–isooctane (2) system to the following model for gE:![]()
The system temperature of interest is 200°C. After a literature search, the only vapor–liquid equilibrium data at this temperature that you can fi nd is:
For the pure components, the Antoine constants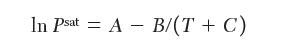
where Psat sat is in torr (1/760 atm) and T is in K, liquid densities, and second virial coeffi cients are as follows: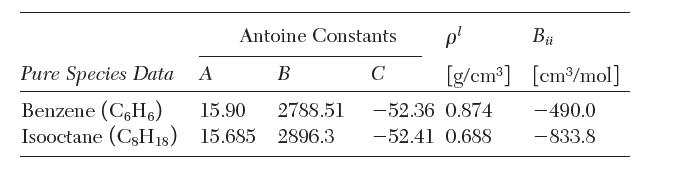
(a) Using only the data given above, as accurately as you can, fi nd the constants A and B (in J/mol). State any assumptions that you make.
(b) Is it ever possible for benzene and isooctane to split into two partially miscible liquid phases?
Explain. If so, in what temperature range would you start to look for partial miscibility?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





