A substance has the following heating curve: (a) What is the melting point of the substance? (b)
Question:
A substance has the following heating curve:
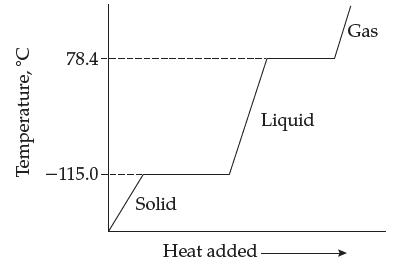
(a) What is the melting point of the substance?
(b) What is its freezing point?
(c) What is its boiling point?
(d) At what temperature does the vapor condense?
(e) Why isn’t the temperature increasing as the substance is being heated at the horizontal portions of the curve?
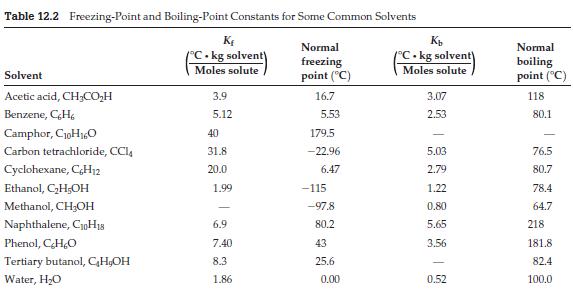
(f) According to Table 12.2, what is this substance?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:





