Adjust the At equilibrium rate meters of Problem 14.41 to make them show a reaction behavior that
Question:
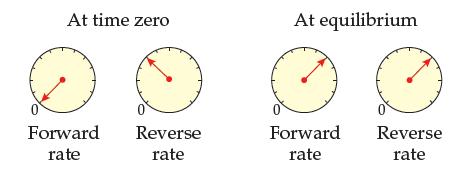
Adjust the “At equilibrium” rate meters of Problem 14.41 to make them show a reaction behavior that is possible. Explain why it is possible.
Data from Problem 14.41
Is the following behavior possible for a reaction run at constant temperature? Explain your answer.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:





